Preparation and Characterization of Esterified Bamboo Flour by an In Situ Solid Phase Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
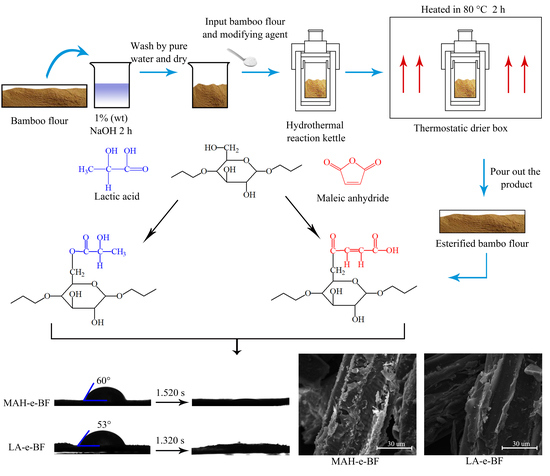
2.2. Preparation of Esterified BF
2.3. Properties and Characterization
2.3.1. Fourier-Transform Infrared Spectroscopic Analysis
2.3.2. Determination of Esterification Degree
2.3.3. Determination of Water Absorption
2.3.4. Contact Angle Measurements
2.3.5. Scanning Electron Microscopic Analysis
2.3.6. X-ray Diffraction Analysis
2.3.7. Thermogravimetric Analysis
3. Results and Discussion
3.1. In Situ Solid Phase Polymerization Confirmation
3.2. Morphology Change of Esterified BF
3.3. Water Resistance of Esterified BF
3.4. Crystalline Structural Changes of Esterified BF
3.5. Thermal Performance Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Y.J.; Xu, B.; Zhang, Q.S.; Jiang, S.X. Present situation and the countermeasure analysis of bamboo timber processing industry in China. J. For. Eng. 2016, 1, 2–7. [Google Scholar]
- Huang, Y.; Fei, B.; Wei, P.; Zhao, C. Mechanical properties of bamboo fiber cell walls during the culm development by nanoindentation. Ind. Crop. Prod. 2016, 92, 102–108. [Google Scholar] [CrossRef]
- Nugroho, N.; Ando, N. Development of structural composite products made from bamboo II: fundamental properties of laminated bamboo lumber. J. Wood Sci. 2011, 47, 237–242. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind. Crop. Prod. 2014, 56, 128–136. [Google Scholar] [CrossRef]
- Tung, N.H.; Yamamoto, H.; Matsuoka, T.; Fujii, T. Effect of surface treatment on interfacial strength between bamboo fiber and PP resin. JSME Int. J. Ser. A 2004, 47, 561–565. [Google Scholar] [CrossRef]
- Ashori, A. Wood–plastic composites as promising green-composites for automotive industries. Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Tokoro, R.; Vu, D.M.; Okubo, K.; Tanaka, T.; Fujii, T.; Fujiura, T. How to improve mechanical properties of polylactic acid with bamboo fibers. J. Mater. Sci. 2008, 43, 775–787. [Google Scholar] [CrossRef]
- Li, W.; Qiao, X.; Sun, K.; Chen, X. Effect of electron beam irradiation on the silk fibroin fiber/poly (ε-caprolactone) composite. J. Appl. Polym. Sci. 2009, 113, 1063–1069. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable polymers/bamboo fiber bio-composite with bio-based coupling agent. Compos. Part A 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Plackett, D.; Andersen, T.L.; Pedersen, W.B.; Nielsenc, L. Biodegradable composites based on L-polylactide and jute fibres. Compos. Sci. Technol. 2003, 63, 1287–1296. [Google Scholar] [CrossRef]
- Avella, M.; Bogoeva-Gaceva, G.; Buzoarovska, A.; Errico, E.M.; Gentile, G.; Grozdanov, A. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)-based biocomposites reinforced with kenaf fibers. J. Appl. Polym. Sci. 2007, 104, 3192–3200. [Google Scholar] [CrossRef]
- Liu, L.; Yang, W.; Yang, M. The solid-phase grafting of maleic anhydride onto microcrystalline cellulose. Polym. Mater. Sci. Eng. 2010, 26, 127–129. [Google Scholar]
- Liu, Q.S.; Tong, Z.; Peng, W.; Guo, L. Preparation and characterization of activated carbon from bamboo by microwave-induced phosphoric acid activation. Ind. Crops Prod. 2010, 31, 233–238. [Google Scholar] [CrossRef]
- Xue, Z.H.; Zhao, G.J. Influence of different treatments of on wood crystal properties. J. Northwest For. Univ. 2007, 22, 169–171. [Google Scholar]
- Dontulwar, J.R.; Borikar, D.K.; Gogte, B.B. An esteric polymer synthesis and its characterization using starch, glycerol and maleic anhydride as precursor. Carbohydr. Polym. 2006, 65, 207–210. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J.; Wang, N. Preparation and properties of biodegradable poly (propylene carbonate)/thermoplastic dried starch composites. Carbohydr. Polym. 2008, 71, 229–234. [Google Scholar] [CrossRef]
- Tay, S.H.; Pang, S.C.; Chin, S.F. Facile synthesis of starch-maleate monoesters from native sago starch. Carbohydr. Polym. 2012, 88, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, P.K.; Kumar, R. Influence of chemical treatments on the mechanical and water absorption properties of bamboo fiber composites. J. Reinf. Plast. Compos. 2011, 30, 73–85. [Google Scholar] [CrossRef]
- Zeng, J.B.; Jiao, L.; Li, Y.D.; Srinivasan, M.; Li, T.; Wang, Y.Z. Bio-based blends of starch and poly (butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, T.; Chang, P.R.; Lia, K.; Wu, Q. Starch composites reinforced by bamboo cellulosic crystals. Bioresour. Technol. 2010, 101, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and characterization of PLA-based green composites: A review. J. Thermoplast. Compos. Mater. 2014, 27, 52–81. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Y.; Pei, X.; He, X.; Li, P.; Wu, Y.; Zuo, Y. Preparation and Characterization of Esterified Bamboo Flour by an In Situ Solid Phase Method. Polymers 2018, 10, 920. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080920
Geng Y, Pei X, He X, Li P, Wu Y, Zuo Y. Preparation and Characterization of Esterified Bamboo Flour by an In Situ Solid Phase Method. Polymers. 2018; 10(8):920. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080920
Chicago/Turabian StyleGeng, Yaqi, Xiaohan Pei, Xiaoyu He, Ping Li, Yiqiang Wu, and Yingfeng Zuo. 2018. "Preparation and Characterization of Esterified Bamboo Flour by an In Situ Solid Phase Method" Polymers 10, no. 8: 920. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10080920