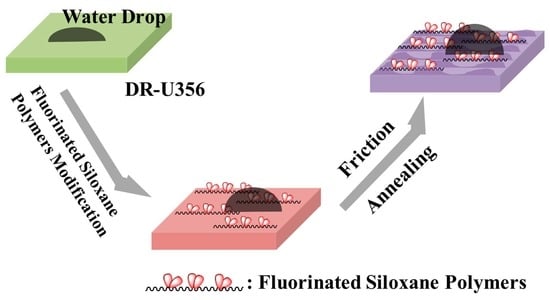
A Novel Synthetic UV-Curable Fluorinated Siloxane Resin for Low Surface Energy Coating
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Synthesis of Monomers
2.2.1. Synthesis of Hydroxyl-Containing Polydimethylsiloxane (OH-PDMS)
2.2.2. Modification of Fluorinated Intermediaries (OH-G01, OH-G05, and OH-G06B)
2.2.3. Synthesis of Graft Fluorinated Siloxane Polymers (Si-G01, Si-G05, and Si-G06B)
2.3. Preparation of Samples
2.3.1. Resin Formulation
2.3.2. Preparation Method
2.4. Characterization
2.5. UV Curing Operation
3. Results and Discussion
3.1. Synthesis and Characterization of Novel UV-Curable Fluorinated Siloxane Polymers
3.1.1. Characterization of Silicone Intermediates (H-PDMS, OH-PDMS, and EPDMS)
3.1.2. Characterization of Modified Fluorine-Containing Acrylic Acid
3.2. UV-Curing Behavior
3.3. Surface Characterization
3.3.1. X-Ray Photoelectron Analysis
3.3.2. Contact Angle and Surface Energy of the Resin
3.3.3. Friction Recovering Properties
3.4. Differential Scanning Calorimetry (DSC)
3.5. Chemical Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Golovin, K.; Boban, M.; Mabry, J.M.; Tuteja, A. Designing self-healing superhydrophobic surfaces with exceptional mechanical durability. ACS Appl. Mater. Interface 2017, 9, 11212–11223. [Google Scholar] [CrossRef] [PubMed]
- Sagisaka, M.; Narumi, T.; Niwase, M.; Narita, S.; Ohata, A.; James, C.; Yoshizawa, A.; Givenchy, E.T.D.; Guittard, F.; Alexander, S.; et al. Hyperbranched hydrocarbon surfactants give fluorocarbon-like low surface energies. Langmuir 2014, 30, 6057–6063. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Smith, G.N.; James, C.; Rogers, S.E.; Guittard, F.; Sagisaka, M.; Eastoe, J. Low-surface energy surfactants with branched hydrocarbon architectures. Langmuir 2014, 30, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional superhydrophobic surfaces templated from innately microstructured hydrogel matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef] [PubMed]
- Buerkle, M.; Asai, Y. Thermal conductance of Teflon and Polyethylene: Insight from an atomistic, single-molecule level. Sci. Rep. 2017, 7, 41898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, L.; Lackner, J.M.; Kot, M.; Major, B. Bio-tribological properties and microstructure characterization of the polytetrafluorethylene (PTFE) coatings on polyaryletheretherketone (PEEK) substrate. Tribol. Int. 2016, 104, 309–320. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, B.; Huang, L.; Ma, D.; Jiao, Z.; Xie, Y.; Tan, S.; Cai, X. The utilization of carbon nitride to reinforce the mechanical and thermal properties of UV-curable waterborne polyurethane acrylate coatings. Prog. Org. Coat. 2015, 89, 35–41. [Google Scholar] [CrossRef]
- Król, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Li, J.; Weng, R. Preparation of nano-SiO2/amino-modified polysiloxane hybrid superhydrophobic coating and thermal-stability characterization. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 35–39. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, W.; Gao, N.; Wang, H.; Su, K. Synthesis and properties of a novel UV-cured fluorinated siloxane graft copolymer for improved surface, dielectric and tribological properties of epoxy acrylate coating. Appl. Surf. Sci. 2013, 284, 683–691. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, W.; Wang, H.; Su, K.; Hou, G. Synthesis and characterization of novel fluorinated siloxane star-like copolymer with short perfluoroalkyl chain and used for modification the epoxy resin. J. Fluor. Chem. 2014, 157, 63–72. [Google Scholar] [CrossRef]
- Cappelletto, E.; Callone, E.; Campostrini, R.; Girardi, F.; Maggini, S.; Volpe, C.D. Hydrophobic siloxane paper coatings: The effect of increasing methyl substitution. J. Sol-Gel Sci. Technol. 2012, 62, 441–452. [Google Scholar] [CrossRef]
- Tao, C.; Li, X.; Liu, B.; Zhang, K.; Zhao, Y.; Zhu, K.; Yuan, X. Highly icephobic properties on slippery surfaces formed from polysiloxane and fluorinated POSS. Prog. Org. Coat. 2017, 103, 48–59. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Qiu, X.; Aytuǧ, T.; Kidder, M.K.; Messman, J.M.; Sauers, I. Nonfunctionalized polydimethyl siloxane superhydrophobic surfaces based on hydrophobic−hydrophilic interactions. Langmuir 2011, 27, 2953–2957. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Luo, Z.H. Novel superhydrophobic silica/poly (siloxane-fluoroacrylate) hybrid nanoparticles prepared via surface-initiated ATRP and their surface properties: The effects of polymerization conditions. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 174–183. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Oss, C.J.V.; Wu, W.; Docoslis, A.; Giese, R.F. The interfacial tensions with water and the Lewis acid–base surface tension parameters of polar organic liquids derived from their aqueous solubilities. Colloids Surf. B Biointerfaces 2001, 20, 87–91. [Google Scholar] [PubMed]
- Wu, M.; An, N.; Li, Y.; Sun, J. Layer-by-layer assembly of fluorine-free polyelectrolyte–surfactant complexes for the fabrication of self-healing superhydrophobic films. Langmuir 2016, 32, 12361–12369. [Google Scholar] [CrossRef] [PubMed]













| Si-G01 (g) | Si-G05 (g) | Si-G06B (g) | DR-U356 (g) | Acetone (g) | Initiator (g) | |
|---|---|---|---|---|---|---|
| SG01-01 | 0.1 | × | × | 9.9 | 2.0 | 0.4 |
| SG01-02 | 0.2 | × | × | 9.8 | 2.0 | 0.4 |
| SG01-03 | 0.3 | × | × | 9.7 | 2.0 | 0.4 |
| SG01-04 | 0.4 | × | × | 9.6 | 2.0 | 0.4 |
| SG01-05 | 0.5 | × | × | 9.5 | 2.0 | 0.4 |
| SG01-10 | 1.0 | × | × | 9.0 | 2.0 | 0.4 |
| SG05-01 | × | 0.1 | × | 9.9 | 2.0 | 0.4 |
| SG05-02 | × | 0.2 | × | 9.8 | 2.0 | 0.4 |
| SG05-03 | × | 0.3 | × | 9.7 | 2.0 | 0.4 |
| SG05-04 | × | 0.4 | × | 9.6 | 2.0 | 0.4 |
| SG05-05 | × | 0.5 | × | 9.5 | 2.0 | 0.4 |
| SG05-10 | × | 1.0 | × | 9.0 | 2.0 | 0.4 |
| SG06B-01 | × | × | 0.1 | 9.9 | 2.0 | 0.4 |
| SG06B-02 | × | × | 0.2 | 9.8 | 2.0 | 0.4 |
| SG06B-03 | × | × | 0.3 | 9.7 | 2.0 | 0.4 |
| SG06B-04 | × | × | 0.4 | 9.6 | 2.0 | 0.4 |
| SG06B-05 | × | × | 0.5 | 9.5 | 2.0 | 0.4 |
| SG06B-10 | × | × | 1.0 | 9.0 | 2.0 | 0.4 |
| Theory (%) | Measurement (%) | |
|---|---|---|
| SG05-01 | 0.148 | 2.86 |
| SG05-03 | 0.444 | 2.21 |
| SG05-05 | 0.740 | 2.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Yang, H.; Liang, H.; Wang, Z.; Dong, J.; Xiong, L.; Zhou, J.; Ke, J.; Xu, X.; Xi, W. A Novel Synthetic UV-Curable Fluorinated Siloxane Resin for Low Surface Energy Coating. Polymers 2018, 10, 979. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090979
Zhu C, Yang H, Liang H, Wang Z, Dong J, Xiong L, Zhou J, Ke J, Xu X, Xi W. A Novel Synthetic UV-Curable Fluorinated Siloxane Resin for Low Surface Energy Coating. Polymers. 2018; 10(9):979. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090979
Chicago/Turabian StyleZhu, Chunfang, Haitao Yang, Hongbo Liang, Zhengyue Wang, Jun Dong, Lei Xiong, Jianping Zhou, Junjun Ke, Xi Xu, and Weixian Xi. 2018. "A Novel Synthetic UV-Curable Fluorinated Siloxane Resin for Low Surface Energy Coating" Polymers 10, no. 9: 979. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090979