Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide
Abstract
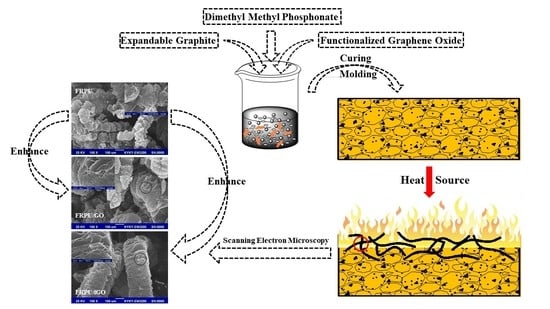
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Testing
3. Results and Discussion
3.1. Flame Retardancy of RPUF Specimens
3.2. Thermal Stability of RPUF Specimens
3.3. Decomposition Activity Energies
3.4. FT-IR Analysis of the Residues Heated to Specific Temperatures
3.5. Digital Photos and SEM Images of Char Residue
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Usta, N. Investigation of fire behavior of rigid polyurethane foams containing fly ash and intumescent flame retardant by using a cone calorimeter. J. Appl. Polym. Sci. 2012, 124, 3372–3384. [Google Scholar] [CrossRef]
- Verdolotti, L.; Lavorgna, M.; Maio, E.D.; Iannace, S. Hydration-induced reinforcement of rigid polyurethane–cement foams: The effect of the co-continuous morphology on the thermal-oxidative stability. Polym. Degrad. Stab. 2013, 98, 64–72. [Google Scholar] [CrossRef]
- Gao, L.P.; Zheng, G.Y.; Zhou, Y.H.; Hu, L.H.; Feng, G.D.; Xie, Y.L. Synergistic effect of expandable graphite, melamine polyphosphate and layered double hydroxide on improving the fire behavior of rosin-based rigid polyurethane foam. Ind. Crop. Prod. 2013, 50, 638–647. [Google Scholar] [CrossRef]
- Danowska, M.; Piszczyk, L.; Strankowski, M.; Gazda, M.; Haponiuk, J.T. Rigid polyurethane foams modified with selected layered silicate nanofillers. J. Appl. Polym. Sci. 2013, 130, 2272–2281. [Google Scholar] [CrossRef]
- Vitkauskienė, I.; Makuška, R.; Stirna, U.; Cabulis, U. Thermal Properties of Polyurethane Polyisocyanurate Foams Based on Poly (ethylene terephthalate) Waste. Mater. Sci. 2011, 17, 249–253. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Properties of Rigid PolyurethanePolyisocyanurate Foams Modified with the Selected Filllers. J. Appl. Polym. Sci. 2008, 115, 2460–2469. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Checchin, M. Influence of different flame retardants on fire behaviour of modified PIR/PUR polymers. Polym. Degrad. Stab. 2001, 74, 475–479. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weill, E.D. Thermal decomposition, combustion and fire-retardancy of polyurethanes—A review of the recent literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Tang, Z.; Maroto-Valer, M.M.; Andresen, J.M.; Miller, J.W.; Listemann, M.L.; McDaniel, P.L.; Morita, D.K.; Furlan, W.R. Thermal degradation behavior of rigid polyurethane foams prepared with different fire retardant concentrations and blowing agents. Polymer 2002, 43, 6471–6479. [Google Scholar] [CrossRef]
- Chung, Y.; Kim, Y.; Kim, S. Flame retardant properties of polyurethane produced by the addition of phosphorous containing polyurethane oligomers (II). J. Ind. Eng. Chem. 2009, 15, 888–893. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.Y.; Liu, X.M.; Tang, J.H.; Li, Z.M. Flame-retardant and mechanical properties of high-density rigid polyurethane foams filled with decabrominated dipheny ethane and expandable graphite. J. Appl. Polym. Sci. 2009, 111, 2372–2380. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, R.; Wang, X.; Wang, B.B.; Song, L.; Hu, Y.; Gong, X.L. Enhancement of flame retardant performance of bio-based polylactic acid composites with the incorporation of aluminum hypophosphite and expanded graphite. J. Macromol. Sci. A. 2013, 50, 255–269. [Google Scholar] [CrossRef]
- Kuranska, M.; Cabulis, U.; Auguscik, M.; Prociak, A.; Ryszkowska, J.; Kirpluks, M. Bio-based polyurethane-polyisocyanurate composites with an intumescent flame retardant. Polym. Degrad. Stab. 2016, 127, 11–19. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on paraffin/high density polyethylene composites. Renew. Energy. 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Zhang, P.; Song, L.; Lu, H.; Wang, J.; Hu, Y. The influence of expanded graphite on thermal properties for paraffin/high density polyethylene/chlorinated paraffin/antimony trioxide as a flame retardant phase change material. Energy Convers. Manag. 2010, 51, 2733–2737. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Zeltins, V.; Stiebra, L.; Avots, A. Rigid polyurethane foam thermal insulation protected with mineral intumescent mat. Autex Res. J. 2014, 14, 259–269. [Google Scholar] [CrossRef]
- Li, G.; Yuan, J.B.; Zhang, Y.H.; Zhang, N.; Liew, K.M. Microstructure and mechanical performance of graphene reinforced cementitious composites. Compos. Part A Appl. Sci. Manuf. 2018, 114, 188–195. [Google Scholar] [CrossRef]
- Feng, W.; Qin, M.; Lv, P.; Li, J.; Feng, Y. A three-dimensional nanostructure of graphite intercalated by carbon nanotubes with high cross-plane thermal conductivity and bending strength. Carbon 2014, 77, 1054–1064. [Google Scholar] [CrossRef]
- Omidvar, A.; RashidianVaziri, M.R.; Jaleh, B. Enhancing the nonlinear optical properties of graphene oxide by repairing with palladium nanoparticles. Physica E 2018, 103, 239–245. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, Y.; Xu, N.; Zhao, P.; Zhan, R.; She, J.; Chen, J.; Deng, S. Pinhole evolution of few-layer graphene during electron tunneling and electron transport. Carbon 2018, 139, 688–694. [Google Scholar] [CrossRef]
- Bettina, D.; Katen, A.W.; Rolf, M.; Bernhard, S. Flame-Retardancy Properties of Intumescent Ammonium Poly (Phosphate) and Mineral Filler Magnesium Hydroxide in Combination with Graphene. Polymers 2014, 6, 2875–2895. [Google Scholar]
- Bao, C.; Guo, Y.; Yuan, B.; Hu, Y.; Song, L. Functionalized graphene oxide for fire safety applications of polymers: A combination of condensed phase flame retardant strategies. J. Mater. Chem. 2012, 22, 23057–23063. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Gudarzi, M.M. Intumescent flame retardant polyurethane/reduced graphene oxide composites with improved mechanical, thermal, and barrier properties. J. Mater. Sci. 2014, 49, 243–254. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.F.; Yue, L.N.; Chai, Z.H. The flame retardancy of epoxy resin including the modified graphene oxide and ammonium polyphosphate. Combust Sci. Technol. 2018, 190, 1126–1140. [Google Scholar]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron–graphene and melamine salt of pentaerythritol phosphate on flame retardant thermoplastic polyurethane. Polym. Adv. Technol. 2016, 27, 1508–1516. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.F.; Zhou, X. A flame retardant rigid polyurethane foam system including functionalized graphene oxide. Polym. Compos. 2018. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Chen, J.; Rong, M.; Ruan, W.; Zhang, M. Interfacial enhancement of nano-SiO2/polypropylene composites. Compos. Sci. Technol. 2009, 69, 252–259. [Google Scholar] [CrossRef]
- Chen, X.L.; Ma, C.Y.; Jiao, C.M. Aluminum hypophosphite in combination with expandable graphite as a novel flame retardant system for rigid polyurethane foams. Polym. Adv. Technol. 2014, 25, 1034–1043. [Google Scholar]
- Xu, W.Z.; Liu, L.; Wang, S.Q.; Hu, Y. Synergistic effect of expandable graphite and aluminum hypophosphite on flame-retardant properties of rigid polyurethane foam. J. Appl. Polym. Sci. 2015, 132, 42842. [Google Scholar] [CrossRef]
- Wang, S.; Qian, L.; Xin, F. The Synergistic Flame-Retardant Behaviors of Pentaerythritol Phosphate and Expandable Graphite in Rigid Polyurethane Foams. Polym. Compos. 2018, 39, 329–336. [Google Scholar] [CrossRef]






| Sample | Polyether Polyol (g) | Isocyanate (g) | EG (phr) | DMMP (phr) | GO (phr) | fGO (phr) | LOI (%) | UL-94 Rating |
|---|---|---|---|---|---|---|---|---|
| RPUF | 50 | 50 | – | – | – | 19.0 | No rating | |
| FRPU | 50 | 50 | 7.5 | 2.5 | – | 26.5 | V-1 | |
| FRPU/GO | 50 | 50 | 7.5 | 2.5 | 0.25 | 27.5 | V-0 | |
| FRPU/fGO | 50 | 50 | 7.5 | 2.5 | 0.25 | 28.1 | V-0 |
| Sample | Air | Nitrogen | ||||||
|---|---|---|---|---|---|---|---|---|
| Tini (°C) | Tmax (°C) | Residue at 700 °C (%) | Tini (°C) | Tmax (°C) | Residue at 700 °C (%) | |||
| T1max | T2max | T3max | ||||||
| RPUF | 281 | 134 | 317 | 541 | 0.5 | 276 | 349 | 15.9 |
| FRPU | 257 | 161 | 320 | 549 | 7.0 | 256 | 345 | 20.3 |
| FRPU/GO | 260 | 163 | 319 | 548 | 9.5 | 269 | 346 | 22.7 |
| FRPU/fGO | 269 | 164 | 321 | 551 | 12.5 | 278 | 347 | 24.4 |
| Sample | Heating Rate, Φ (°C·min−1) | Tm (°C) | Activation Energy, Ea (kJ·mol−1) |
|---|---|---|---|
| RPUF | 5 | 326.6 | 130 |
| 10 | 347.0 | ||
| 15 | 349.3 | ||
| 20 | 358.0 | ||
| FRPU | 5 | 325.6 | 128 |
| 10 | 338.2 | ||
| 15 | 346.1 | ||
| 20 | 357.7 | ||
| FRPU/GO | 5 | 330.2 | 170 |
| 10 | 338.0 | ||
| 15 | 348.2 | ||
| 20 | 353.3 | ||
| FRPU/fGO | 5 | 330.3 | 191 |
| 10 | 339.3 | ||
| 15 | 346.7 | ||
| 20 | 351.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, J.; Gao, M. Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide. Polymers 2019, 11, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010078
Chen X, Li J, Gao M. Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide. Polymers. 2019; 11(1):78. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010078
Chicago/Turabian StyleChen, Xuexi, Junfei Li, and Ming Gao. 2019. "Thermal Degradation and Flame Retardant Mechanism of the Rigid Polyurethane Foam Including Functionalized Graphene Oxide" Polymers 11, no. 1: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11010078