A Novel Inherently Flame-Retardant Composite Based on Zinc Alginate/Nano-Cu2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
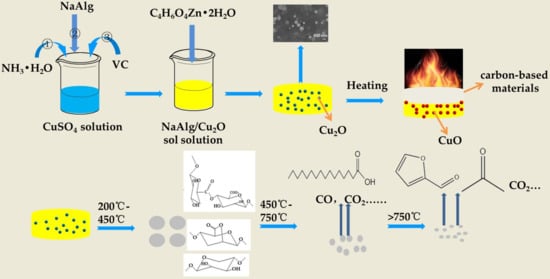
2.2. Preparation of ZnAlg
2.3. Preparation of ZnAlg/Cu2O Composites
2.4. Measurements
3. Results and Discussion
3.1. Characterizations
3.2. Thermal Stability
3.3. Flame Retartancy
3.4. Combustion Behavior
3.5. Flame-Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym. Degrad. Stab. 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.; Cooper, P.; Lawson, M.; Triffitt, J.; Barralet, J. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohl, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT-Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Shalumon, K.; Anulekha, K.; Nair, S.V.; Nair, S.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Boil. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.C.; Zhang, C.J.; Guo, Y.; Zhu, P.; Wang, D.Y. Effect of manganese and cobalt ions on flame retardancy and thermal degradation of bio-based alginate films. J. Mater. Sci. 2016, 51, 1052–1065. [Google Scholar] [CrossRef]
- Kong, Q.-S.; Wang, B.-B.; Ji, Q.; Xia, Y.-Z.; Guo, Z.-X.; Yu, J. thermal degradation and flame retardancy of calcium alginate fibers. Chin. J. Polym. Sci. 2009, 27, 807. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhang, C.; Ji, H.; Zhu, P. The Flame Retardancy, Thermal Properties, and Degradation Mechanism of Zinc Alginate Films. J. Macromol. Sci. Part B 2014, 53, 1074–1089. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Wang, F.; Tan, L.; Xia, Y. Effects of divalent metal ions on the flame retardancy and pyrolysis products of alginate fibres. Polym. Degrad. Stab. 2012, 97, 1034–1040. [Google Scholar] [CrossRef]
- Chan, L.; Jin, Y.; Heng, P. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef]
- Straccia, M.C.; D’Ayala, G.G.; Romano, I.; Laurienzo, P. Novel zinc alginate hydrogels prepared by internal setting method with intrinsic antibacterial activity. Carbohydr. Polym. 2015, 125, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, E.; Ke, W. Effect of nanoparticles on the improvement in fire-resistant and anti-ageing properties of flame-retardant coating. Surf. Coat. Technol. 2006, 200, 5706–5716. [Google Scholar] [CrossRef]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as Effective Flame Retardants for Natural and Synthetic Textile Polymers: Application, Mechanism, and Optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Murphy, C.J. Solution-Phase Synthesis of Cu2O Nanocubes. Nano Lett. 2003, 3, 231–234. [Google Scholar] [CrossRef]
- Li, C.L.; Li, Y.B.; Delaunay, J.J. A novel method to synthesize highly photoactive Cu2O microcrystalline films for use in photoelectrochemical cells. Acs Appl. Mater. Inter. 2014, 6, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zou, J.J.; Zhang, T.R.; Wang, S.B.; Li, Z.; Wang, L.; Zhang, X.W. Cu2O film via hydrothermal redox approach: Morphology and photocatalytic performance. J. Phys. Chem. C 2014, 118, 16335–16343. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape Evolution and Size-Controllable Synthesis of Cu2O Octahedra and Their Morphology-Dependent Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tsui, L.-K.; Swami, N.; Zangari, G. Photoelectrochemical Stability of Electrodeposited Cu2O Films. J. Phys. Chem. C 2010, 114, 11551–11556. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; Xue, F.; Wang, X.; Li, X.; Guo, L. Chronic effects of six micro/nano-Cu2O crystals with different structures and shapes on Daphnia magna. Environ. Pollut. 2015, 203, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Montazer, M.; Samadi, N. Synthesis of nano Cu2O on cotton: Morphological, physical, biological and optical sensing characterizations. Carbohydr. Polym. 2014, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Kou, S.; Zhang, J.; Tang, X.Y.; Yang, Q.; Yao, B.H. Preparation and characterization of Cu2O nano-particles and their photocatalytic degradation of fluroxypyr. Environ. Technol. 2018, 39, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, X.; Xiao, M.; Huang, Z.; Tan, X. Effect of particle size and morphology on surface thermodynamics and photocatalytic thermodynamics of nano-Cu2O. J. Environ. Chem. Eng. 2017, 5, 4447–4453. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, Q.; Geng, B. Microwave chemical route to self-assembled quasi-spherical Cu2O microarchitectures and their gas-sensing properties. Sens. Actuators B Chem. 2009, 143, 253–260. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, R.Z.; Xiong, S.L.; Liu, Y.K.; Chai, L.L.; Bao, K.Y.; Qian, Y.T. Well-aligned Cu2O nanowire arrays prepared by an ethylene glycol-reduced process. Mater. Chem. Phys. 2009, 114, 213–216. [Google Scholar] [CrossRef]
- Jing, S.; Xing, S.; Wu, Y.; Wang, Y.; Zhao, B.; Zhao, C. Synthesis of octahedral Cu2O microcrystals assisted with mixed cationic/anionic surfactants. Mater. Lett. 2007, 61, 2281–2283. [Google Scholar] [CrossRef]
- Zhang, D.M.; Hu, B.S.; Guan, D.J.; Luo, Z.T. Essential roles of defects in pure graphene/Cu2O photocatalyst. Catal. Commun. 2016, 76, 7–12. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.; Gao, W.; Lu, X. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Zhao, X.; Li, Z.; Li, Q. Surface Coating for Flame Retardancy and Pyrolysis Behavior of Polyester Fabric Based on Calcium Alginate Nanocomposites. Nanomaterials 2018, 8, 875. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhao, X.; Zhang, L.; Li, Q. Highly Efficient flame eetardant Hybrid Composites Based on Calcium Alginate/Nano-Calcium Borate. Polymers 2018, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qi, Y.; Wang, Y.; Xue, Y.; Xu, P.; Li, Z.; Li, Q. Morphology and Thermal Properties of Calcium Alginate/Reduced Graphene Oxide Composites. Polymers 2018, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Ávila, R.; Cadenas-Pliego, G.; Ávila-Orta, C.; Jiménez-Regalado, E.; Hernández-Hernández, E.; Jímenez-Barrera, R.M.; Pérez-Alvarez, M.; Padilla, V.C.; Camacho, O.P. Synthesis of Copper Nanoparticles Using Mixture of Allylamine and Polyallylamine. J. Nanomater. 2015, 2015, 140. [Google Scholar] [CrossRef]
- Sierra-Ávila, R.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; Betancourt-Galindo, R.; Jiménez-Regalado, E.; Jímenez-Barrera, R.M.; Pérez-Alvarez, M.; Martínez-Colunga, J.G. Synthesis of Copper Nanoparticles Coated with Nitrogen Ligands. J. Nanomater. 2014, 2014, 74. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Sierra-Ávila, R.; Ávila-Orta, C.A.; Jiménez-Regalado, E.; Bello, A.M.; González-Morones, P.; Cadenas-Pliego, G. Oxidation of Copper Nanoparticles Protected with Different Coatings and Stored under Ambient Conditions. J. Nanomater. 2018, 2018, 9512768. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.C.; Zhao, X.H.; Deng, Y.J.; Xue, Y.; Li, Q. Flame retardancy and thermal degradation mechanism of calcium alginate/CaCO3 composites prepared via in situ method. J. Therm. Anal. Calorim. 2018, 131, 2167–2177. [Google Scholar] [CrossRef]
- Ma, X.; Li, R.; Zhao, X.; Ji, Q.; Xing, Y.; Sunarso, J.; Xia, Y. Biopolymer composite fibres composed of calcium alginate reinforced with nanocrystalline cellulose. Compos. Part A Appl. Sci. Manuf. 2017, 96, 155–163. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the Mechanism by Which the Formation of Nanocomposites Enhances Thermal Stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Chen, H.-B.; Shen, P.; Chen, M.; Schiraldi, D.A.; Zhao, H.-B. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Tian, G.; Ji, Q.; Xu, D.; Tan, L.; Quan, F.; Xia, Y. The effect of zinc ion content on flame retardance and thermal degradation of alginate fibers. Fibers Polym. 2013, 14, 767–771. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.-J.; Zhao, J.-C.; Guo, Y.; Zhu, P.; Wang, D.-Y. Bio-based barium alginate film: Preparation, flame retardancy and thermal degradation behavior. Carbohydr. Polym. 2016, 139, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-J.; Liu, Y.; Cui, L.; Yan, C.; Zhu, P. Bio-based calcium alginate nonwoven fabrics: Flame retardant and thermal degradation properties. J. Anal. Appl. Pyrolysis 2016, 122, 13–23. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, Y.; Wang, J.; Liu, X.; Song, T.; Yang, X.; Hao, J. Spray-Drying-Assisted Layer-by-Layer Assembly of Alginate, 3-Aminopropyltriethoxysilane, and Magnesium Hydroxide Flame Retardant and Its Catalytic Graphitization in Ethylene–Vinyl Acetate Resin. ACS Appl. Mater. Interfaces 2018, 10, 10490–10500. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Wang, J.; Zhu, P.; Zhao, J.; Zhang, C.; Guo, Y.; Jin, X. Thermal degradation and pyrolysis behavior of aluminum alginate investigated by TG-FTIR-MS and Py-GC-MS. Polym. Degrad. Stab. 2015, 118, 59–68. [Google Scholar] [CrossRef]
- Taghiyari, H.R. Fire-retarding properties of nano-silver in solid woods. Wood Sci. Technol. 2012, 46, 939–952. [Google Scholar] [CrossRef]










| Sample | LOI (%) | UL-94 | Time to Ignition (s) | Time to Flameout (s) | THR (MJ/m2) | PHRR (kW/m2) | TSR (m2/m2) | Residue (%) |
|---|---|---|---|---|---|---|---|---|
| ZnAlg | 49 | V-0 | 22 | 387 | 29.71 | 162.31 | 274.28 | 51.29 |
| ZnAlg/Cu2O | 58 | V-0 | 37 | 610 | 25.71 | 83.18 | 288.75 | 56.74 |
| Molecular Structure Name of Compound | T = 250 °C | T = 450 °C | T = 750 °C | ||||
|---|---|---|---|---|---|---|---|
| Time | Area | Time | Area | Time | Area | ||
 | carbon dioxide | 1.87 | 2.03 | 1.57 | 71.37 | 1.57 | 74.46 |
 | water | 1.57 | 68.52 | - | - | - | - |
 | carbon monoxide | 1.15 | 18.36 | 1.26 | 6.74 | - | - |
 | furfural | - | - | 4.23 | 3.88 | 4.23 | 5.67 |
 | acetone | - | - | - | - | 1.78 | 3.54 |
 | 2,3-butanedione | - | - | - | - | 2.06 | 1.24 |
 | 2-furancarboxaldehyde,5-methyl | - | - | 5.62 | 0.27 | 5.62 | 0.74 |
 | furan,2-methyl- | - | - | - | - | 2.14 | 0.30 |
 | benzene | - | - | - | - | 2.53 | 0.50 |
 | acetic acid | - | - | 2.20 | 1.03 | - | - |
 | 4-cyclopentene-1,3-dione | - | - | - | - | 4.83 | 0.37 |
 | 1,3-cyclopentadiene | - | - | - | - | 1.89 | 0.14 |
 | 2-furancarboxaldehyde,5-methyl | - | - | - | - | 5.12 | 0.73 |
 | styrene | - | - | - | - | 4.90 | 0.12 |
 | phenol | - | - | - | - | 6.04 | 0.19 |
 | n-hexadecanoic acid | 12.38 | 1.68 | 12.38 | 2.25 | - | - |
 | 2-butenal, (E)- | - | - | - | - | 2.44 | 0.27 |
 | toluene | - | - | - | - | 3.51 | 0.34 |
 | 2-furanmethanol | - | - | 7.03 | 0.46 | - | - |
 | propanoicacid,2-oxo- | - | - | 1.79 | 1.33 | - | - |
 | 3-furaldehyde | - | - | 4.03 | 0.48 | - | - |
 | acetoin | - | - | 2.91 | 0.23 | - | - |
 | undecanoic acid | - | - | 8.26 | 0.29 | - | - |
 | 2h-pyran,3,4-dihydro-4-hydroxy | - | - | 11.26 | 0.30 | - | - |
 | 9-hexadecenoicacid | - | - | 9.60 | 0.91 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Shao, P.; Zhang, Q.; Cheng, W.; Li, Z.; Li, Q. A Novel Inherently Flame-Retardant Composite Based on Zinc Alginate/Nano-Cu2O. Polymers 2019, 11, 1575. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101575
Xu P, Shao P, Zhang Q, Cheng W, Li Z, Li Q. A Novel Inherently Flame-Retardant Composite Based on Zinc Alginate/Nano-Cu2O. Polymers. 2019; 11(10):1575. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101575
Chicago/Turabian StyleXu, Peng, Peiyuan Shao, Qing Zhang, Wen Cheng, Zichao Li, and Qun Li. 2019. "A Novel Inherently Flame-Retardant Composite Based on Zinc Alginate/Nano-Cu2O" Polymers 11, no. 10: 1575. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101575