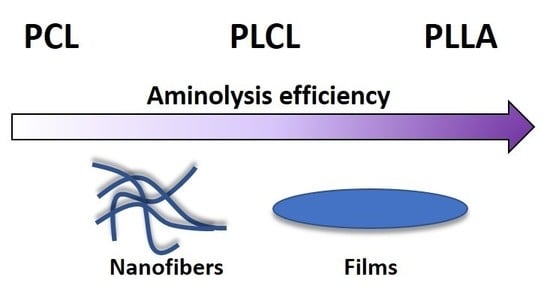
Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PCL, PLCL, and PLLA Nanofibrous Scaffolds and Films
2.3. Aminolysis Treatment
2.4. Ninhydrin Assay
2.5. ATR–FTIR
2.6. WAXS
2.7. Mechanical Testing
2.8. Water Contact Angle
3. Results
3.1. Ninhydrin Staining of Free Amino Groups
3.2. Molecular Structure from ATR–FTIR Analysis
3.3. Supermolecular Structure (Crystallinity) from WAXS
3.4. Mechanical Properties
3.5. Water Contact Angle
4. Discussion
- Various ratios of ester to alkyl groups being the lowest for PCL and the highest for PLLA.
- Different crystallinity, being the highest for PCL and the lowest for PLLA. It is known that the availability of ester groups for aminolysis depends on the supermolecular organization, being lower for the crystal phase than for the amorphous one.
- Different rates of NH2 recombination after aminolysis, being dependent on the position of the aminolysis temperature in relation to glass transition temperature. It is anticipated that in the case of PCL with glass transition temperature far below aminolysis temperature, the free amine recombination rate should be much higher than for PLCL with Tg = 32–42 °C, and particularly for PLLA, for which aminolysis is performed below glass transition temperature.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Z.; Mao, Z.; Gao, C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloid Surf. B Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef]
- Vasita, R.; Shanmugam, I.K.; Katt, D.S. Improved Biomaterials for Tissue Engineering Applications: Surface Modification of Polymers. Curr. Top. Med. Chem. 2008, 8, 341–353. [Google Scholar] [CrossRef]
- Wang, Y.X.; Robertson, J.L.; Spillman, W.B., Jr.; Claus, R.O. Effects of the Chemical Structure and the Surface Properties of Polymeric Biomaterials on Their Biocompatibility. Pharm. Res. 2004, 21, 1362–1373. [Google Scholar] [CrossRef]
- Budnicka, M.; Szymaniak, M.; Kołbuk, D.; Ruśkowski, P.; Gadomska-Gajadhur, A. Biomineralization of poly-l-lactide spongy bone scaffolds obtained by freeze-extraction method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Kołbuk, D.; Urbanek, O.; Denis, P.; Choińska, E. Sonochemical coating as an effective method of polymeric nonwovens functionalization. J. Biomed. Mater. Res. Part A 2019, in press. [Google Scholar]
- Jeznach, O.; Gajc, M.; Korzeb, K.; Kłos, A.; Orliński, K.; Stępień, R.; Krok-Borkowicz, M.; Rumian, Ł.; Pietryga, K.; Reczyńska, K.; et al. New calcium-free Na2O-Al2O3-P2O5 bioactive glasses with potential applications in bone tissue engineering. J. Am. Ceram. Soc. 2018, 101, 602–611. [Google Scholar] [CrossRef]
- Heljak, M.K.; Moczulska-Heljak, M.; Choińska, E.; Chlanda, A.; Kosik-Kozioł, A.; Jaroszewicz, T.; Jaroszewicz, J.; Swieszkowski, W. Micro and nanoscale characterization of poly(DL-lactic-co-glycolic acid) films subjected to the L929 cells and the cyclic mechanical load. Micron 2018, 115, 64–72. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W.; Han, S.; Bunpetch, V.; Zhao, K.; Liu, C.; Yin, Z.; Ouyang, H. Cell-material interactions in tendon tissue engineering. Acta Biomater. 2018, 70, 1–11. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef]
- Kooshki, H.; Ghollasi, M.; Halabian, R.; Kazemi, N.M. Osteogenic differentiation of preconditioned bone marrow mesenchymal stem cells with lipopolysaccharide on modified poly-l-lactic-acid nanofibers. J. Cell. Physiol. 2019, 234, 5343–5353. [Google Scholar] [CrossRef] [PubMed]
- Wismayer, K.; Mehrban, N.; Bowen, J.; Birchall, M. Improving cellular migration in tissue-engineered laryngeal scaffolds. J. Laryngol. Otol. 2019, 133, 135–148. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Z.; Gao, C. Aminolysis-based surface modification of polyesters for biomedical applications. RSC Adv. 2013, 3, 2509–2519. [Google Scholar] [CrossRef]
- De Luca, A.C.; Terenghi, G.; Downes, S. Chemical surface modification of poly-ε-caprolactone improves Schwann cell proliferation for peripheral nerve repair. J. Tissue Eng. Regen. Med. 2014, 8, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhu, Y.; Zhang, J.; Hu, P. Surface modification of polyhydroxyalkanoates by ion implantation. Characterization and cytocompatibility improvement. Polym. J. 2003, 35, 148–154. [Google Scholar] [CrossRef]
- Birhanu, G.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Dusti Telgerd, M. An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci Eng. C Mater. Biol. Appl. 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Ai, J.; Soleimani, M.; Verdi, J.; Mohammad Tavangar, S.; Sadroddiny, E.; Massumi, M.; Mahmoud Hashemi, S. Improved human endometrial stem cells differentiation into functional hepatocyte-like cells on a glycosaminoglycan/collagen-grafted polyethersulfone nanofibrous scaffold. J. Biomed. Mater. Res. B 2017, 105, 2516–2529. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X. Effects of functionalization of PLGA-[Asp-PEG]n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. J. Biomed. Mater. Res. A 2014, 102, 4526–4535. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Krithica, N.; Natarajan, V.; Madhan, B.; Sehgal, P.K.; Mandal, A.B. Type I Collagen Immobilized Poly(caprolactone) Nanofibers: Characterization of Surface Modification and Growth of Fibroblasts. Adv. Eng. Mater. 2012, 4, B149–B154. [Google Scholar] [CrossRef]
- Cao, L.; Yu, Y.; Wang, J.; Werkmeister, J.A.; McLean, K.M.; Liu, C. 2-N, 6-O-sulfated chitosan-assisted BMP-2 immobilization of PCL scaffolds for enhanced osteoinduction. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 74, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.F.; Sahagian, K.; Huang, F.; Xu, K.; Patel, S.; Li, S. Comparison of plasma and chemical modifications of poly-L-lactide-co-caprolactone scaffolds for heparin conjugation. Biomed. Mater. 2017, 12, 065004. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, L.; Huang, L.; Lai, Y. Evaluation of ethylenediamine-modified nanofibrillated cellulose/chitosan composites on adsorption of cationic and anionic dyes from aqueous solution. Carbohydr. Polym. 2016, 151, 1115–1119. [Google Scholar] [CrossRef]
- Ohe, T.; Yoshimura, Y. Reaction of PET fibers with ethylenediamine in a water solution containing surfactans. Sen’i Gakkaishi 2012, 68, 253–258. [Google Scholar] [CrossRef]
- Fukatsu, K. Mechanical Properties of Poly(ethylene terephtalate) Fibers Imparted Hydrophilicity with Aminolysis. J. Appl. Polym. Sci. 1992, 45, 2037–2042. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, K.; Zhou, Y.; Ye, Z.; Tan, W.S. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(ε-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 193–202. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of Biomacromolecules onto Aminolyzed Poly(L-lactic acid) toward Acceleration of Endothelium Regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 897–907. [Google Scholar] [CrossRef]
- Amirian, J.; Lee, S.Y.; Lee, B.T. Designing of Combined Nano and Microfiber Network by Immobilization of Oxidized Cellulose Nanofiber on Polycaprolactone Fibrous Scaffold. J. Biomed. Nanotechnol. 2016, 12, 1864–1875. [Google Scholar] [CrossRef]
- Song, M.-J.; Amirian, J.; Linh, N.T.B.; Lee, B.-T. Bone morphogenetic protein-2 immobilization on porous. PCL-BCP-Col composite scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45186. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted PCL nanofibrous scaffold: Promising ECM for bone tissue engineering. Eur. Polym. J. 2015, 71, 490–509. [Google Scholar] [CrossRef]
- Kosmala, A.; Fitzgerald, M.Z.; Moore, E.C.; Stam, F. Evaluation of a Gelatin Modified Poly(ɛ-Caprolactone) Film as a Scaffold for Lung Disease. Anal. Lett. 2017, 50, 219–232. [Google Scholar] [CrossRef]
- Patel, J.J.; Flanagan, C.L.; Hollister, S.J. Bone Morphogenetic Protein-2 Adsorption onto Poly-e-caprolactone Better Preserves Bioactivity In Vitro and Produces More Bone In Vivo than Conjugation Under Clinically Relevant Loading Scenarios. Tissue Eng. Part C Methods 2015, 21, 489–498. [Google Scholar] [CrossRef]
- Stevens, J.S.; De Luca, A.C.; Downes, S.; Terenghi, G.; Schroeder, S.L.M. Immobilisation of cell-binding peptides on poly-ε-caprolactone (PCL) films: A comparative XPS study of two chemical surface functionalisation methods. Surf. Interface Anal. 2014, 46, 673–678. [Google Scholar] [CrossRef]
- Pellegrino, L.; Cocchiola, R.; Francolini, I.; Lopreiato, M.; Piozzi, A.; Zanoni, R.; Scotto d’Abusco, A.; Martinelli, A. Taurine grafting and collagen adsorption on PLLA films improve human primary chondrocyte adhesion and growth. Colloids Surf. B Biointerfaces 2017, 158, 643–649. [Google Scholar] [CrossRef]
- Xu, F.J.; Yang, X.C.; Li, C.Y.; Yang, W.T. Functionalized Polylactide Film Surfaces via Surface-Initiated ATRP. Macromolecules 2011, 44, 2371–2377. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA Nanofibrous Scaffolds Coated with Graphene Oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Yang, Z.; Kim, G.; Chen, H.; Ge, Z. A Viscoelastic Chitosan-Modified Three-Dimensional Porous Poly(L-lactide-co-ε-caprolactone) scaffold for cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 405–424. [Google Scholar] [CrossRef]
- Zhu, Y.; Chian, K.S.; Chan-Park, M.B.; Mhaisalkar, P.S.; Ratner, B.D. Protein bonding on biodegradable poly(L-lactide-co-caprolactone). Biomaterials 2006, 27, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Leong, M.F.; Ong, W.F.; Chan-Park, M.B.; Chian, K.S. Esophageal epithelium regeneration on fibronectin grafted poly(L-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Lee, J.; Shin, Y.M.; Shin, H.J.; Madhurakat Perikamana, S.K.; Park, S.H.; Kim, S.W.; Shin, H. Hybrid-spheroids incorporating ECM like engineered fragmented fibers potentiate stem cell function by improved cell/cell and cell/ECM. Acta Biomater. 2017, 64, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.G.; Lo Giudice, M.C.; Vermonden, T.; Leeuwenburgh, S.C.; Jansen, J.A.; van den Beucken, J.J.; Yang, F. A Top-Down Approach for the Preparation of Highly Porous PLLA Microcylinders. ACS Biomater. Sci. Eng. 2016, 2, 2099–2107. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Polini, A.; Azami, Z.; Leeuwenburgh, S.C.G.; Jansen, J.A.; Yang, F.; van den Beucken, J.J.J.P. Incorporation of PLLA micro-fillers for mechanical reinforcement of calcium-phosphate cement. J. Mech. Behav. Biomed. Mater. 2017, 71, 286–294. [Google Scholar] [CrossRef]
- Xie, Z.; Buschle-Diller, G. Functionalized Poly(L-lactide) nanoparticles from electrospun nanofibers. J. Biomater. Sci. Polym. Ed. 2011, 22, 1331–1341. [Google Scholar] [CrossRef]
- Polini, A.; Petre, D.G.; Iafisco, M.; de Lacerda Schickert, S.; Tampieri, A.; van den Beucken, J.; Leeuwenburgh, S.C.G. Polyester fibers can be rendered calcium phosphate-binding by surface functionalization with bisphosphonate groups. J. Biomed. Mater. Res. A 2017, 105, 2335–2342. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Kołbuk, D.; Guimond-Lischer, S.; Sajkiewicz, P.; Maniura-Weber, K.; Fortunato, G. The Effect of Selected Electrospinning Parameters on Molecular Structure of Polycaprolactone Nanofibers. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 365–377. [Google Scholar] [CrossRef]
- Li, J.; Xiao, P.; Li, H.; Zhang, Y.; Xue, F.; Luo, B.; Huang, S.; Shang, Y.; Wen, H.; de Claville, C.J.; et al. Crystalline structures and crystallization behaviors of poly (L-lactide) in poly (L-lactide)/graphene nanosheet composites. Polym. Chem. 2015, 6, 3988–4002. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, G.J.; Fu, Q.; Lei, J.; Jiang, W.; Hsiao, B.S.; Li, Z.M. Formation of shish-kebabs in injection-molded poly(L-lactic acid) by application of an intense flow field. ACS Appl. Mater. Interfaces 2012, 4, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Tensile Strength in Relation to Molecular Weight of High Polymers. J. Am. Chem. Soc. 1945, 67, 2048–2050. [Google Scholar] [CrossRef]
- Bersted, B.H.; Anderson, T.G. Influence of molecular weight and molecular weight distribution on the tensile properties of amorphous polymers. J. Appl. Polym. Sci. 1990, 39, 499–514. [Google Scholar] [CrossRef]
- Yang, Z.; Zhengwei, M.; Huayu, S.; ChangYou, G. In-depth study on aminolysis of poly(e-caprolactone): Back to the fundamentals. Sci. China Chem. 2012, 55, 2419–2427. [Google Scholar] [CrossRef]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly (ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Pol. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Ganjaliniaa, A.; Akbaria, S.; Solouk, A. PLLA scaffolds surface-engineered via poly(propylene imine) dendrimers for improvement on its biocompatibility/controlled pH biodegradability. Appl. Surf. Sci. 2017, 394, 446–456. [Google Scholar] [CrossRef]
- Bakry, A.; Darwish, M.S.A.; El Naggar, A.M.A. Assembling of hydrophilic and cytocompatible three-dimensional scaffolds based on aminolyzed poly(L-lactide) single crystals. New J. Chem. 2018, 42, 16930–16939. [Google Scholar] [CrossRef]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Krivkina, E.O.; Mironov, A.V.; Burago, A.Y.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; et al. Bioabsorbable Bypass Grafts Biofunctionalised with RGD Have Enhanced Biophysical Properties and Endothelialisation Tested In vivo. Front. Pharmacol. 2016, 7, 136. [Google Scholar] [CrossRef]
- Holmes, S.A. Aminolysis of poly(ethylene terephthalate) in aqueous amine and amine vapour. J. Appl. Polym. Sci. 1996, 61, 255–260. [Google Scholar] [CrossRef]
- Shimizu, J.; Okui, N.; Kikutani, T. Fine Structure and Physical Properties of Fibers Melt-Spun at High-Speeds from Various Polymers. In High-Speed Fiber Spinning, Science and Engineering Aspects; Ziabicki, A., Kawai, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1985; Chapter 15. [Google Scholar]
- Shimizu, J.; Kikutani, T.; Takaku, A. Structure Developments in High-Speed Spinning. In Proceedings of the International Symposium Fiber Science and Technology, Hakone, Japan, 20–24 August 1985. [Google Scholar]
- Perez, G. Some effects of the rheological properties of polyethylene terephthalate) on spinning line profile and structure developed in high speed spinning. In High-Speed Fiber Spinning, Science and Engineering Aspects; Ziabicki, A., Kawai, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1985; Chapter 12. [Google Scholar]
- Perez, G.; Jung, E. High Speed Melt Spinning: Fiber Structure and Properties. In Proceedings of the International Symposium Fiber Science and Technology, Hakone, Japan, 20–24 August 1985. [Google Scholar]
- Kameoka, J.; Craighead, H.G. Fabrication of oriented polymeric nanofibers on planar surfaces by electrospinning. Appl. Phys. Lett. 2003, 83, 371–373. [Google Scholar] [CrossRef]
- Kameoka, J.; Orth, R.; Yang, Y.; Czaplewski, D.; Mathers, R.; Coates, G.W.; Craighead, H.G. A scanning tip electrospinning source for deposition of oriented nanofibers. Nanotechnology 2003, 14, 1124–1129. [Google Scholar] [CrossRef]
- Buer, A.; Ugbolue, S.C.; Warner, S.B. Electrospinning and Properties of Some Nanofibers. Text. Res. J. 2001, 71, 323–328. [Google Scholar] [CrossRef]
- Filatov, Y.; Budyk, A.; Kirichenko, V. Connecticut, Electrospinning of Micro- and Nanofibers: Fundamentals and Applications in Separation and Filtration Process; NH Begell House: New York, NY, USA, 2007. [Google Scholar]
- Fennessey, S.F.; Farris, R.J. Fabrication of aligned and molecularly oriented electrospun polyacrylonitrile nanofibers and the mechanical behaviour of their twisted yarns. Polymer 2004, 45, 4217–4225. [Google Scholar] [CrossRef]
- Behler, K.; Havel, M.; Gogotsi, Y. New solvent for polyamides and its application to the electrospinning of polyamides 11 and 12. Polymer 2007, 48, 6617–6621. [Google Scholar] [CrossRef]
- Wang, X.; Cao, J.; Hu, Z.; Pan, W.; Liu, Z. Jet Shaping Nanofibers and the Collection of Nanofiber Mats in Electrospinning. J. Mater. Sci. Technol. 2006, 22, 536. [Google Scholar]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Tsou, S.Y.; Lin, H.S. Brill transition of nylon-6 in electrospun nanofibers. Colloid Polym. Sci. 2012, 290, 1799–1809. [Google Scholar] [CrossRef]
- Piai, J.F.; da Silva, M.A.; Martins, A.; Torres, A.B.; Faria, S.; Reis, R.L.; Muniz, E.C.; Neves, N.M. Chondroitin sulfate immobilization at the surface of electrospun nanofiber meshes for cartilage tissue regeneration approaches. Appl. Surf. Sci. 2017, 403, 112–125. [Google Scholar] [CrossRef]
- Molisak-Tolwinska, H.; Wencel, A.; Figaszewski, Z. The Effect of Hydrophilization of Polypropylene Membranes with Alcohols on Their Transport Properties. J. Macromol. Sci. A 1998, 35, 857–865. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Yang, F.; van den Beucken, J.J.J.P.; Jansen, J.A. Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative; Khang, G., Ed.; Pan Stanford Publishing: Singapore, 2017; Chapter 18; p. 489. [Google Scholar]
- Shahidi, S.; Wiener, J.; Ghoranneviss, M. Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; IntechOpen: London, UK, 2013; Chapter 2; p. 44. [Google Scholar]
- Causa, F.; Battista, E.; Della, M.R.; Guarnieri, D.; Iannone, M.; Netti, P.A. Surface Investigation on Biomimetic Materials to Control Cell Adhesion: The Case of RGD conjugation on PCL. Langmuir 2010, 26, 9875–9884. [Google Scholar] [CrossRef] [PubMed]
- Monnier, A.; Al Tawil, E.; Nguyen, Q.T.; Valleton, J.M.; Fatyeyeva, K.; Deschrevel, B. Functionalization of poly (lactic acid) scaffold surface by aminolysis and hyaluronan immobilization: How it affects mesenchymal stem cell proliferation. Eur. Polym. J. 2018, 107, 202–217. [Google Scholar] [CrossRef]
- Pavlinec, J.; Lazar, M. Cross-linking of poly (methyl methacrylate) by aminolysis of ester functions with diamines. J. Appl. Polym. Sci 1995, 55, 39–45. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films. Polymers 2019, 11, 1669. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101669
Jeznach O, Kolbuk D, Sajkiewicz P. Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films. Polymers. 2019; 11(10):1669. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101669
Chicago/Turabian StyleJeznach, Oliwia, Dorota Kolbuk, and Paweł Sajkiewicz. 2019. "Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films" Polymers 11, no. 10: 1669. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11101669