Thermo/pH Responsive Star and Linear Copolymers Containing a Cholic Acid-Derived Monomer, N-Isopropylacrylamide and Acrylic Acid: Synthesis and Solution Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Synthesis of the CTAs 1 to 3
3.2. Synthesis and Characterization of MacroCTAs 1 to 3 (Also, Polymerization of CAE in the Presence of CTAs)
3.3. Chain Extension Polymerization of the MacroCTAs Containing PCAE with NIPAM or NIPAM/AAc
3.4. Aqueous Solution Properties of Block Copolymers
3.4.1. Thermosensitivity Behavior of Block Copolymers Synthesized without Acrylic Acid
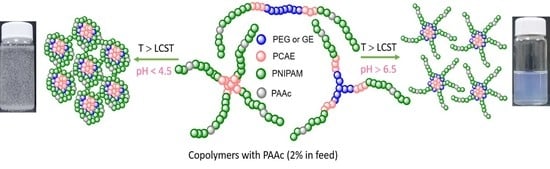
3.4.2. pH/Thermosensitivity Behavior of Block Copolymers Containing Acrylic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Pojer, C.; Zenz, R.; Lammert, F.; Stieger, B.; Meier, P.J.; Zatloukal, K.; et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 2001, 121, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, J.; Kolehmaineu, E. Bile Acids as Building Blocks of Supramolecular Hosts. Molecules 2001, 6, 21–46. [Google Scholar] [CrossRef]
- Benrebouh, A.; Zhang, Y.; Zhu, X.X. Hydrophilic polymethacrylates containing cholic acid-ethylene glycol derivatives as pendant groups. Macromol. Rapid Commun. 2000, 21, 685–690. [Google Scholar] [CrossRef]
- Benrebouh, A.; Avoce, D.; Zhu, X.X. Thermo- and pH-sensitive polymers containing cholic acid derivatives. Polymer 2001, 42, 4031–4038. [Google Scholar] [CrossRef]
- Liu, H.; Avoce, D.; Song, Z.; Zhu, X.X. N-Isopropylacrylamide copolymers with acrylamide and methacrylamide derivatives of cholic acid: Synthesis and characterization. Macromol. Rapid Commun. 2001, 22, 675–680. [Google Scholar] [CrossRef]
- Zhu, X.X.; Nichifor, M. Polymeric materials containing bile acids. Acc. Chem. Res. 2002, 35, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.J.; Zhu, X.X. Polymers made of bile acids: From soft to hard biomaterials. Can. J. Chem. 2016, 94, 659–666. [Google Scholar] [CrossRef]
- Zhang, K.; Gia, Y.G.; Tsai, I.H.; Strandman, S.; Ren, L.; Hong, L.; Zhang, G.; Guan, Y.; Zhang, Y.; Zhu, X.X. Bitter-Sweet polymeric micelles formed by block copolymers from glucosamine and cholic acid. Biomacromolecules 2017, 18, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Wei, H.; Li, S.L.; Feng, J.; Nie, J.; Zhang, X.Z.; Zhuo, R.X. Fabrication of star-shaped, thermo-sensitive poly(N-isopropylacrylamide)-cholic acid-poly(ε-caprolactone) copolymers and their self-assembled micelles as drug carriers. Polymer 2008, 49, 3965–3972. [Google Scholar] [CrossRef]
- Feng, J.; Wen, W.; Jia, Y.G.; Liu, S.; Guo, J. pH-responsive micelles assembled by three-armed degradable block copolymers with a cholic acid core for drug controlled-release. Polymers 2019, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Hill, D.J.T.; Whittaker, A.K. Solution Properties of Star and Linear Poly (N-isopropylacrylamide. Macromolecules 2006, 39, 8379–8388. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Effect of molecular architecture of hydrophobically modified poly(N-isopropylacrylamide) on the formation of thermoresponsive core-shell micellar drug carriers. J. Control. Release 1998, 53, 119–130. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Lin, P.Y.; Chen, T.; Kuo, S.W. Temperature-, pH- and CO2-Sensitive Poly (N-isopropylacrylamide-co-acrylic acid) Copolymers with High Glass Transition Temperatures. Polymers 2016, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hoffman, A.S. Temperature-induced phase transition behaviors of random vs. graft copolymers of N-isopropylacrylamide and acrylic acid. Macromol. Rapid Commun. 1995, 16, 175–182. [Google Scholar] [CrossRef]
- Kim, J.C.; Bae, S.K.; Kim, J.D. Temperature-sensitivity of liposomal lipid bilayers mixed with poly (N-isopropylacrylamide-co-acrylic acid). J. Biochem. 1997, 121, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.S. Effect of pH on the lower critical solution temperatures of random copolymers of N-isopropylacrylamide and acrylic acid. Eur. Polym. J. 1999, 35, 795–801. [Google Scholar] [CrossRef]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly (N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Preparation of a mini-library of thermo-responsive star (NVCL/NVP-VAc) polymers with tailored properties using a hexafunctional xanthate RAFT agent. Polymers 2018, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Boschmann, D.; Vana, P. Z-RAFT Star Polymerizations of Acrylates: Star Coupling via Intermolecular Chain Transfer to Polymer. Macromolecules 2007, 40, 2683–2693. [Google Scholar] [CrossRef]
- Mayadunne, R.; Jeffery, J.; Moad, G.; Rizzardo, E. Living Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization): Approaches to Star Polymers. Macromolecules 2003, 36, 1505–1513. [Google Scholar] [CrossRef]
- Qu, Y.; Chang, X.; Chen, S.; Zhang, W. In situ synthesis of thermoresponsive 4-arm star block copolymer nano-assemblies by dispersion RAFT polymerization. Polym. Chem. 2017, 8, 3485–3496. [Google Scholar] [CrossRef]
- Cao, M.; Han, G.; Duan, W.; Zhang, W. Synthesis of multi-arm star thermo-responsive polymers and topology effect on phase transition. Polym. Chem. 2018, 9, 2625–2633. [Google Scholar] [CrossRef]
- Herfurth, C.; Malo de Molina, P.; Wieland, C.; Rogers, S.; Gradzielski, M.; Laschewsky, A. One-step RAFT synthesis of well-defined amphiphilic star polymers and their self-assembly in aqueous solution. Polym. Chem. 2012, 3, 1606–1617. [Google Scholar] [CrossRef]
- Pal, S.; Roy, S.G.; De, P. Synthesis via RAFT polymerization of thermo- and pH-responsive random copolymers containing cholic acid moieties and their self-assembly in water. Polym. Chem. 2014, 5, 1275–1284. [Google Scholar] [CrossRef]
- Kang, J.S.; DeLuca, P.P.; Lee, K.C. Emerging pegylated drugs. Expert Opin. Emerg. Drugs 2009, 14, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cheng, S.X.; Zhang, X.Z.; Zhuo, R.X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Star-Shaped Copolymers Based on Poly(N-vinylcaprolactam) and their Use as Nanocarriers of Methotrexate. J. Aust. Chem. 2017, 70, 1291–1301. [Google Scholar] [CrossRef]
- Lee, J.H.; Matsumoto, H.; Fujii, S.; Takahashi, R.; Sakurai, K. Monodisperse micelles composed of poly (ethylene glycol) attached surfactants: Platonic nature in a macromolecular aggregate. Soft Matter. 2019, 15, 5371–5374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Q.; Hu, G.; Xu, X.H.; Kang, S.M.; Lui, N.; Wu, Z.Q. Synthesis of Redox-Responsive Core Cross-Linked Micelles Carrying Optically Active Helical Poly (phenyl isocyanide) Arms and Their Applications in Drug Delivery. ACS Macro Lett. 2018, 7, 1073–1079. [Google Scholar] [CrossRef]
- Lu, M.; Khine, Y.Y.; Chen, F.; Cao, C.; Garvey, C.J.; Lu, H.; Stenzel, M. Sugar Concentration and Arrangement on the Surface of Glycopolymer Micelles Affect the Interaction with Cancer Cells. Biomacromolecules 2019, 20, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, J.; Tang, J.; Shen, Y.; Guo, W.; Zhou, M.; Wang, R.; Jiang, N.; Gan, Z.; Yu, Q. Tumor Specific and Renal Excretable Star-like Triblock Polymer–Doxorubicin Conjugates for Safe and Efficient Anticancer Therapy. Biomacromolecules 2018, 19, 2849–2862. [Google Scholar] [CrossRef] [PubMed]
- Ree, B.J.; Satoh, Y.; Jin, K.S.; Isono, T.; Kim, T.W.J.; Kakuchi, T.; Satoh, T.; Ree, M. Well-defined and stable nanomicelles self-assembled from brush cyclic and tadpole copolymer amphiphiles: A versatile smart carrier platform. NPG Asia Materials 2017, 9, e453. [Google Scholar] [CrossRef] [Green Version]
- Kermagoret, A.; Fustin, C.A.; Bourguignon, M.; Detrembleur, C.; Jérôme, C.; Debuigne, A. One-pot controlled synthesis of double thermoresponsive N-vinylcaprolactam-based copolymers with tunable LCSTs. Polym. Chem. 2013, 4, 2575–2583. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, B.F.; Chu, J.H.; Lui, N.; Wu, Z.Q. Facile Synthesis of Optically Active and Thermoresponsive Star Block Copolymers Carrying Helical Polyisocyanide Arms and Their Thermo-Triggered Chiral Resolution Ability. ACS Macro Lett. 2018, 7, 127–131. [Google Scholar] [CrossRef]









| CTA | MacroCTA | Molar Ratio [CAE]/[CTA]/[AIBN] | Time (min) | Conv. a (%) | Mntheob (g/mol) | MnGPCc (g/mol) | Ɖc | MnNMRd (g/mol) |
|---|---|---|---|---|---|---|---|---|
| 1 | PCAE3-b-PEG45-b-PCAE3 | 74/5/1 | 300 | 65 | 7572 | 6036 | 1.10 | 5502 |
| 1 | PCAE5-b-PEG45-b-PCAE5 | 69/5/1 | 360 | 55 | 6543 | 8487 | 1.21 | 7769 |
| 2 | (GE7-b-PCAE4)3 | 71/5/1 | 120 | 60 | 6242 | 8519 | 1.06 | 7333 |
| 3 | (PCAE2)4 | 80/5/1 | 90 | 44 | 5032 | 7200 | 1.12 | 5079 |
| MacroCTA | Copolymer | Molar Ratio [NIPAM]/[AAc]/[macroCTA]/[AIBN] | Conv. a (%) | MnNMRb (g/mol) | MnGPCc (g/mol) | Ɖc |
|---|---|---|---|---|---|---|
| 1 | PNIPAM120-b-PCAE3-b-PEG45-b-PCAE3-b-PNIPAM120 | 333/0/2.5/1 | 76 | 33,109 | 25,620 | 1.3 |
| 1 | PNIPAM87-b-PCAE5-b-PEG45-b-PCAE5-b-PNIPAM87 | 323/0/2.5/1 | 45 | 39,299 | 27,400 | 1.4 |
| 1 | PAAc2%-co-PNIPAM147-b-PCAE3-b-PEG45-b-PCAE3-b-PNIPAM147-co-PAAc2% | 326/7/2.5/1 | 72 | 26,664 | 21,440 | 1.3 |
| 2 | (GE7-b-PCAE4-b-PNIPAM79)3 | 980/0/2.5/0.25 | 75 | 34,500 | 32,660 | 1.4 |
| 2 | (GE7-b-PCAE4-b-PNIPAM59-co-PAAc2%)3 | 960/20/2.5/0.25 | 60 | 27,926 | 24,200 | 1.3 |
| 3 | (PCAE2-b-PNIPAM75)4 | 500/0/1/0.25 | 81 | - | 41,400 | 1.37 |
| 3 | (PCAE2-b-PNIPAM93-co-PAAc2%)4 | 490/10/1/0.25 | 73 | - | 49,400 | 1.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Hernández, A.; Cortez-Lemus, N.A. Thermo/pH Responsive Star and Linear Copolymers Containing a Cholic Acid-Derived Monomer, N-Isopropylacrylamide and Acrylic Acid: Synthesis and Solution Properties. Polymers 2019, 11, 1859. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111859
Castro-Hernández A, Cortez-Lemus NA. Thermo/pH Responsive Star and Linear Copolymers Containing a Cholic Acid-Derived Monomer, N-Isopropylacrylamide and Acrylic Acid: Synthesis and Solution Properties. Polymers. 2019; 11(11):1859. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111859
Chicago/Turabian StyleCastro-Hernández, Ana, and Norma Aidé Cortez-Lemus. 2019. "Thermo/pH Responsive Star and Linear Copolymers Containing a Cholic Acid-Derived Monomer, N-Isopropylacrylamide and Acrylic Acid: Synthesis and Solution Properties" Polymers 11, no. 11: 1859. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111859