Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
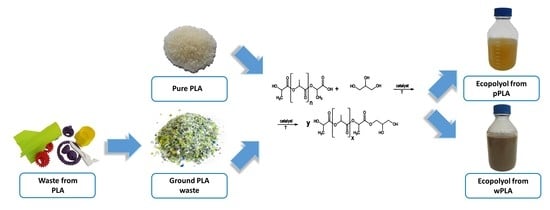
2.2. Glycerolysis of Poly(Lactic Acid)
2.3. Properties of Glycerolysis Products
2.3.1. Physicochemical Tests
2.3.2. Analytical Tests
2.3.3. Spectroscopy Tests
2.3.4. Differential Scanning Calorimetry
2.3.5. Biodegradation Tests
3. Results and discussion
3.1. Physicochemical Properties
3.2. Results of Analytical Tests
3.3. Spectroscopic Analysis
3.4. DSC Analysis
3.5. Susceptibility to Biodegradation
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moubarik, A.; Allal, A.; Pizzi, A.; Charrier, F.; Charrier, B. Characterization of a formaldehyde-free cornstarch-tannin wood adhesive for interior plywood. Eur. J. Wood Wood Prod. 2010, 68, 427–433. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, Ch. J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153. [Google Scholar] [CrossRef] [PubMed]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Lavender Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Env. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef]
- Kijeński, J.; Błędzki, A.K.; Jeziórska, R. Recovery and Recycling of Polymeric Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2011. (In Polish) [Google Scholar]
- Pillai, C.K.S. Challenges for Natural Monomers and Polymers: Novel Design Strategies and Engineering to Develop Advanced Polymers. Des. Monomers Polym. 2010, 13, 87–121. [Google Scholar] [CrossRef]
- Long, J.; Jinwen, Z. Chapter 6. Biodegradable Polymers and Polymer Blends in Handbook of Biopolymers and Biodegradable Plastics. Plast. Des. Libr. 2013, 109–128. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Davies, S.; Kolstad, J.J. The eco-profile for current Ingeo polylactide production. Ind. Biotechnol. 2010, 6, 212. [Google Scholar] [CrossRef]
- Badía, J.D.; Strömberg, E.; Ribes-Greus, A.; Karlsson, S. Assessing the MALDI-TOF MS sample preparation procedure to analyze the influence of thermo-oxidative ageing and thermo-mechanical degradation on poly (Lactide). Eur. Polym. J. 2011, 47, 1416–1428. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ribes-Greus, A. Suitability of Blends from Virgin and Reprocessed Polylactide: Performance and Energy Valorization Kinetics. J. Renew. Mat. 2018, 6, 370–382. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New emerging trends insynthetic biodegradable polymers – Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food pack-aging applications. J. Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.T.; Selke, S.E.M.; Tsuji, H. Poly(Lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- De Smet, M.; Linder, M. A Circular Economy for Plstics: Insights from Research and Innovation to Inform Policy and Funding Decisions; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Calleja, D. Why the “New Plastics Economy” must be a circular economy. Field ACTions Sci. Rep. 2019, 19, 22–27. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Method of Obtaining Polyol Raw Material Based on Polylactide Waste. Polish Patent Application No. P.424629, 20 February 2018. (In Polish). [Google Scholar]
- ISO 758:1976 standard. Liquid Chemical Products for Industrial Use—Determination of Density at 20 Degrees C; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- WT/06/07/PURINOVA plant standard. Determination of Hydroxyl and Acid Values of Polyols; Purinova Ltd.: Bydgoszcz, Poland, 2013. [Google Scholar]
- PN-C-04959:1981 standard. Determination of Water Content by the Karl Fischer Method in Organic and Inorganic Products; Polish Committee for Standardization: Warszawa, Poland, 1981. (In Polish) [Google Scholar]
- ISO 17556:2019 standard. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ISO 11274:2019 standard. Soil Quality—Determination of the Water-Retention Characteristic—Laboratory Methods; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ISO 10390:2005 standard. Soil Quality—Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Borowicz, M. Synthesis and Application of New Bio-Polyols Based On Vegetable Raw Materials for the Production of Bio-Composites in the form of Rigid Polyurethane-Polyisocyanurate Foams. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2019. (In Polish). [Google Scholar]
- Safety Data Sheet of Ingeo® Biopolymer; NatureWorks: Minnetonka, MN, USA, 2015; Available online: https://www.natureworksllc.com/.
- Safety Data Sheet of Anhydrous Glycerol; Chempur: Piekary Śląskie, Poland, 2017; Available online: https://chempur.pl/.
- Czupryński, B. Issues from Chemistry and Technology of Polyurethanes; Wydawnictwo Akademii Bydgoskiej: Bydgoszcz, Poland, 2004. (In Polish) [Google Scholar]
- Prociak, A. New Generation Polyurethane Thermal Insulation Materials; Wydawnictwo Politechniki Krakowskej: Cracow, Poland, 2008. (In Polish) [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014. (In Polish) [Google Scholar]
- Lim, H.; Kim, S.H.; Kim, B.K. Effects of the hydroxyl value of polyol in rigid polyurethane foams. Polym. Adv. Technol. 2008, 19, 1729–1734. [Google Scholar] [CrossRef]
- Lactic Acid—Compound Summary. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactic-acid. (accessed on 29 October 2019).
- Jutrzenka Trzebiatowska, P.; Beneš, H.; Datta, J. Evaluation of the glycerolysis process and valorisation of recovered polyol in polyurethane synthesis. React. Funct. Polym. 2019, 139, 25–33. [Google Scholar] [CrossRef]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer Through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef]
- Datta, J. Effect of glycols used as glycolysis agents on chemical structure and thermal stability of the produced glycolysates. J. Therm. Anal. Calorim. 2012, 109, 517–520. [Google Scholar] [CrossRef]
- Fink, J.K. Chapter 2-Poly(urethane)s in Reactive Polymers Fundamentals and Applications (Second Edition). Plast. Des. Libr. 2013, 49–93. [Google Scholar] [CrossRef]
- Dutta, A.S. Chapter 2-Polyurethane Foam Chemistry in Recycling of Polyurethane Foams. Plastics Design Library 2018, 17–27. [Google Scholar] [CrossRef]
- Spectral Database for Organic Compounds (SDBS). Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (accessed on 25 October 2019).
- FTIR Spectrum of Pure Lactic Acid on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=12682 (accessed on 25 October 2019).
- FTIR Spectrum of Pure Dilactide on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=5501 (accessed on 25 October 2019).
- FTIR Spectrum of Pure Glycerol on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=2517 (accessed on 25 October 2019).
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M. New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers 2019, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- 1HNMR Spectrum of Pure Lactic acid on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr&sdbsno=12682 (accessed on 27 October 2019).
- 1HNMR Spectrum of Pure Dilactide on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr&sdbsno=5501 (accessed on 27 October 2019).
- 1HNMR Spectrum of Pure Glycerol on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=hnmr&sdbsno=2517 (accessed on 27 October 2019).
- 13CNMR Spectrum of Pure Lactic Acid on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=cnmr&sdbsno=12682 (accessed on 28 October 2019).
- 13CNMR Spectrum of Pure Dilactide on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=cnmr&sdbsno=5501 (accessed on 28 October 2019).
- 13CNMR Spectrum of Pure Glycerol on Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=cnmr&sdbsno=2517 (accessed on 28 October 2019).
- Chmiel, E.; Lubczak, J. Oligoetherols and polyurethane foams obtained from metasilicic acid. J. Polym. Bull. 2018, 75, 1579–1596. [Google Scholar] [CrossRef]
- Chmiel, E.; Oliwa, R.; Lubczak, J. Boron-containing non-flammable polyurethane foams. Polym. Plast. Technol. Eng. 2019, 58, 394–404. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Structure analysis and thermal degradation characteristics of bio-based poly(propylene succinate)s obtained by using different catalyst amounts. J. Therm. Anal. Calorim. 2017, 130, 197–206. [Google Scholar] [CrossRef]
- Lin, B.; Yang, L.; Dai, H.; Hou, Q.; Zhang, L. Thermal analysis of soybean oil based polyols. J. Therm. Anal. Calorim. 2009, 95, 977–983. [Google Scholar] [CrossRef]
- Radojčić, D.; Ionescu, M.; Petrović, Z.S. Novel potentially biodegradable polyurethanes from bio-based polyols. Contemp. Mater. 2013, 1, 9–21. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Pattanasuttichonlakul, W.; Sombatsompop, N.; Prapagdee, B. Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int. Biodeter. Biodegr. 2018, 132, 74–83. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Symbol of Compound | wPLA (g) | pPLA (g) | Anhydrous Glycerol (g) | Zinc Stearate (g) |
|---|---|---|---|---|
| wPLA500 | 1000.00 | 0.00 | 500.00 | 2.00 |
| wPLA400 | 1000.00 | 0.00 | 400.00 | 2.00 |
| wPLA300 | 1000.00 | 0.00 | 300.00 | 2.00 |
| pPLA500 | 0.00 | 1000.00 | 500.00 | 2.00 |
| pPLA400 | 0.00 | 1000.00 | 400.00 | 2.00 |
| pPLA300 | 0.00 | 1000.00 | 300.00 | 2.00 |
| Parameter | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| Color (-) | grey | light-green | light-brown | light-yellow | light-yellow | light- yellow |
| Smell (-) | odorless | odorless | odorless | odorless | odorless | odorless |
| Density (g/cm3) | 1.26 ± 0.03 | 1.24 ± 0.05 | 1.26 ± 0.04 | 1.26 ± 0.04 | 1.27 ± 0.03 | 1.28 ± 0.04 |
| Viscosity (mPa·s) | 18,870 ± 350 | 39,900 ± 430 | 138,090 ± 0.04 | 16,420 ± 210 | 41,550 ± 320 | 124,920 ± 760 |
| pH (-) | 6.5 ± 0.1 | 6.5 ± 0.1 | 6.6 ± 0.1 | 6.5 ± 0.1 | 6.6 ± 0.1 | 6.6 ± 0.1 |
| Parameter | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| Hydroxyl value (mg KOH/g) | 543.93 ± 6.12 | 434.36 ± 4.91 | 349.36 ± 4.11 | 563.27 ± 5.23 | 449.96 ± 3.91 | 372.87 ± 3.19 |
| Acid value (mg KOH/g) | 1.37 ± 0.10 | 1.97 ± 0.11 | 1.93 ± 0.09 | 2.06 ± 0.07 | 1.89 ± 0.09 | 1.87 ± 0.10 |
| Water content (% wt.) | 0.17 ± 0.01 | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.10 ± 0.01 | 0.18 ± 0.01 | 0.20 ± 0.02 |
| Element | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| C (%) | 39.68 ± 0.19 | 39.82 ± 0.22 | 40.07 ± 0.12 | 39.59 ± 0.17 | 39.71 ± 0.12 | 39.98 ± 0.15 |
| H (%) | 7.43 ± 0.18 | 7.28 ± 0.17 | 7.16 ± 0.18 | 7.32 ± 0.15 | 7.19 ± 0.21 | 7.05 ± 0.12 |
| O (%) | 52.89 ± 0.15 | 52.90 ± 0.13 | 52.77 ± 0.20 | 53.09 ± 0.22 | 53.10 ± 0.31 | 52.97 ± 0.16 |
| Parameter | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| Mn (g/mol) | 289 | 342 | 384 | 286 | 332 | 376 |
| Mw (g/mol) | 325 | 393 | 452 | 319 | 381 | 438 |
| D (-) | 1.13 | 1.15 | 1.17 | 1.11 | 1.15 | 1.17 |
| f (-) | 3.15 | 3.11 | 2.82 | 3.20 | 3.06 | 2.91 |
| Sample Symbol | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| Tg (°C) | −29.2 | −26.8 | −12.3 | −26.3 | −19.2 | −11.8 |
| Element | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| C (-) | 0.3959 | 0.3971 | 0.3998 | 0.3968 | 0.3982 | 0.4007 |
| H (-) | 0.0732 | 0.0719 | 0.0705 | 0.0743 | 0.0728 | 0.0716 |
| O (-) | 0.5309 | 0.5310 | 0.5297 | 0.5289 | 0.5290 | 0.5277 |
| Sample Symbol | wPLA500 | wPLA400 | wPLA300 | pPLA500 | pPLA400 | pPLA300 |
|---|---|---|---|---|---|---|
| BOD28 (mg/L) | 167.5 | 149.5 | 163.5 | 188.5 | 145.5 | 173.5 |
| TOD (mg/L) | 164.7 | 139.8 | 124.5 | 167.1 | 144.0 | 127.1 |
| Dt (%) | 100 * | 100 * | 100 * | 100 * | 100 * | 100 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Grzybowski, Ł.; Czupryński, B. Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material. Polymers 2019, 11, 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121963
Borowicz M, Paciorek-Sadowska J, Isbrandt M, Grzybowski Ł, Czupryński B. Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material. Polymers. 2019; 11(12):1963. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121963
Chicago/Turabian StyleBorowicz, Marcin, Joanna Paciorek-Sadowska, Marek Isbrandt, Łukasz Grzybowski, and Bogusław Czupryński. 2019. "Glycerolysis of Poly(lactic acid) as a Way to Extend the “Life Cycle” of This Material" Polymers 11, no. 12: 1963. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121963