Green Flexible Polyurethane Foam as a Potent Support for Fe-Si Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PUF
2.3. Synthesis of Fe-Si adsorbent
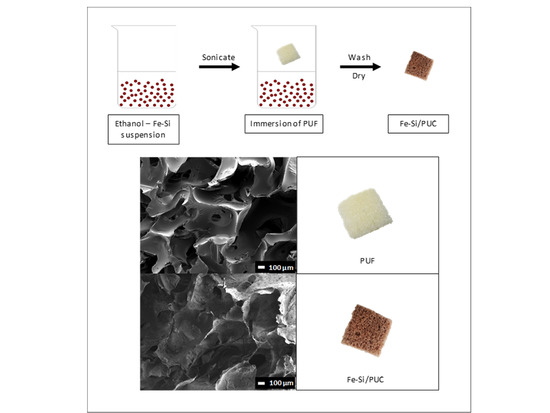
2.4. Impregnation of Fe-Si onto PUF (Fe-Si/PUC)
2.5. Characterisations
2.6. Preliminary Study of Fe-Si/PUC for MB Removal in Aqueous Solution
2.7. Reusability Study of Fe-Si and Fe-Si/PUC
2.8. Quantification of Fe-Si Powder and Fe Residual from Fe-Si/PUC during the Reusability Study
3. Results and Discussion
3.1. Impregnation of Fe-Si onto PUF
3.2. FT-IR
3.3. FESEM-EDX
3.4. BET
3.5. XRD
3.6. Water Uptake
3.7. Compressive Test Analysis
3.8. TGA/DTG
3.9. Preliminary Study of MB Removal in Aqueous Solution
3.10. Reusability Study of Fe-Si and Fe-Si/PUC
3.11. Quantification of Fe-Si Powder and Fe Residual from Fe-Si/PUC during Reusability Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pang, Y.L.; Abdullah, A.Z. Current status of textile industry wastewater management and research progress in Malaysia: A review. Clean-Soil Air Water 2013, 41, 751–764. [Google Scholar] [CrossRef]
- Yaacob, M.R.; Zain, N.F.M.; Zakaria, M.N.; Ismail, M. Environmental management practices in small batik industry in Kelantan, Malaysia. Environ. Manag. 2016, 7, 36–43. [Google Scholar]
- Leyh, R.G.; Kofidis, T.; Strüber, M.; Fischer, S.; Knobloch, K.; Wachsmann, B.; Hagl, C.; Simon, A.R.; Haverich, A. Methylene blue: The drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2003, 125, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R.; Ghaedi, M.; Dashtian, K.; Hajati, S. Sonochemical-assisted synthesis of CuO/Cu2O/Cu nanoparticles as efficient photocatalyst for simultaneous degradation of pollutant dyes in rotating packed bed reactor: LED illumination and central composite design optimization. Ultrason. Sonochem. 2018, 40, 601–610. [Google Scholar] [CrossRef]
- Pu, S.; Xiang, C.; Zhu, R.; Ma, H.; Zinchenko, A.; Chu, W. An efficient heterogeneous Fenton catalyst based on modified diatomite for degradation of cationic dye simulated wastewater. Desalin. Water Treat. 2017, 79, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, L.; Agusti, G.; Bouzeggane, A.; Edouard, D. Adsorption of dye with carbon media supported on polyurethane open cell foam. Catal. Today 2018, 301, 98–103. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Mishra, S.B.; Sheng, L. Iron-included mesoporous silica thin slice as adsorbent and Fenton-like catalyst for the adsorption and degradation of dye in wastewater. Ceram. Int. 2019, 45, 15475–15481. [Google Scholar] [CrossRef]
- Egodawatte, S.; Datt, A.; Burns, E.A.; Larsen, S.C. Chemical insight into the adsorption of chromium(III) on iron oxide/mesoporous silica nanocomposites. Langmuir 2015, 31, 7553–7562. [Google Scholar] [CrossRef]
- Pham, A.L.-T.; Lee, C.; Doyle, F.M.; Sedlak, D.L. A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 2009, 43, 8930–8935. [Google Scholar] [CrossRef] [Green Version]
- Popova, M.; Ristić, A.; Lazar, K.; Maučec, D.; Vassileva, M.; Novak Tušar, N. Iron-functionalized silica nanoparticles as a highly efficient adsorbent and catalyst for toluene oxidation in the gas phase. ChemCatChem 2013, 5, 986–993. [Google Scholar] [CrossRef]
- Zeng, L. A method for preparing silica-containing iron(III) oxide adsorbents for arsenic removal. Water Res. 2003, 37, 4351–4358. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crop. Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Pinto, E.R.P.; Barud, H.S.; Silva, R.R.; Palmieri, M.; Polito, W.L.; Calil, V.L.; Ribeiro, S.J.L.; Messaddeq, Y. Transparent composites prepared from bacterial cellulose and castor oil based polyurethane as substrates for flexible OLEDs. J. Mater. Chem. C 2015, 3, 11581–11588. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crop. Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Zulkifli, N.N.; Badri, K.H.; Nor, M.A.A.M.; Amin, K.A.M. Palm kernel oil-based polyurethane film: Biocompatibility and antibacterial activity studies. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2017; p. 020005. [Google Scholar] [CrossRef]
- Cao, X.; James Lee, L.; Widya, T.; Macosko, C. Polyurethane/clay nanocomposites foams: Processing, structure and properties. Polymer 2005, 46, 775–783. [Google Scholar] [CrossRef]
- Francés, A.B.; Bañón, M.V.N. Effect of silica nanoparticles on polyurethane foaming process and foam properties. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; p. 012020. [Google Scholar]
- Silva, M.C.; Takahashi, J.A.; Chaussy, D.; Belgacem, M.N.; Silva, G.G. Composites of rigid polyurethane foam and cellulose fiber residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar] [CrossRef]
- Lefebvre, L.; Kelber, J.; Jierry, L.; Ritleng, V.; Edouard, D. Polydopamine-coated open cell polyurethane foam as an efficient and easy-to-regenerate soft structured catalytic support (S 2 CS) for the reduction of dye. J. Environ. Chem. Eng. 2017, 5, 79–85. [Google Scholar] [CrossRef]
- Nyari, N.L.D.; Fernandes, I.A.; Bustamante-Vargas, C.E.; Steffens, C.; Oliveira, D.; Zeni, J.; Rigo, E.; Dallago, R.M. In situ immobilization of Candida antarctica B lipase in polyurethane foam support. J. Mol. Catal. B Enzym. 2016, 124, 52–61. [Google Scholar] [CrossRef]
- Pardieu, E.; Chau, N.T.T.; Dintzer, T.; Romero, T.; Favier, D.; Roland, T.; Edouard, D.; Jierry, L.; Ritleng, V. Polydopamine-coated open cell polyurethane foams as an inexpensive, flexible yet robust catalyst support: Proof of concept. Chem. Commun. 2016, 52, 4691–4693. [Google Scholar] [CrossRef]
- Sayed, M.; Burham, N. Removal of cadmium (II) from aqueous solution and natural water samples using polyurethane foam/organobentonite/iron oxide nanocomposite adsorbent. Int. J. Environ. Sci. Technol. 2018, 15, 105–118. [Google Scholar] [CrossRef]
- Cui, C.; Li, L.; Li, M. Improvement of lipase activity by synergistic immobilization on polyurethane and its application for large-scale synthesizing vitamin A. palmitate. Prep. Biochem. Biotechnol. 2019, 49, 485–492. [Google Scholar] [CrossRef]
- Zia, K.M.; Barikani, M.; Zuber, M.; Bhatti, I.A.; Barmar, M. Surface characteristics of polyurethane elastomers based on chitin/1,4-butane diol blends. Int. J. Biol. Macromol. 2009, 44, 182–185. [Google Scholar] [CrossRef]
- Chen, J.-H.; Ruckenstein, E. Solvent-stimulated surface rearrangement of polyurethanes. J. Colloid Interface Sci. 1990, 135, 496–507. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Sánchez-Polo, M.; Polo, A.M.S.; Rivera-Utrilla, J.; Berber-Mendoza, M.S. Tinidazole degradation assisted by solar radiation and iron-doped silica xerogels. Chem. Eng. J. 2018, 344, 21–33. [Google Scholar] [CrossRef]
- Kara, F.; Aksoy, E.A.; Yuksekdag, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef]
- Phuengprasop, T.; Sittiwong, J.; Unob, F. Removal of heavy metal ions by iron oxide coated sewage sludge. J. Hazard. Mater. 2011, 186, 502–507. [Google Scholar] [CrossRef]
- Fabrizioli, P.; Bürgi, T.; Burgener, M.; van Doorslaer, S.; Baiker, A. Synthesis, structural and chemical properties of iron oxide–silica aerogels. J. Mater. Chem. 2002, 12, 619–630. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Dubey, N.C.; Subair, R.; Choudhury, S.; Stamm, M. Enhanced hydrophilic and antifouling polyacrylonitrile membrane with polydopamine modified silica nanoparticles. RSC Adv. 2016, 6, 4448–4457. [Google Scholar] [CrossRef]








| BET (m2/g) | Fe Content (%) | Si Content (%) | |
|---|---|---|---|
| Fe-Si adsorbent | 349 | 14.9 | 26.8 |
| Water Uptake (%) | Compressive Stress (MPa) | Compressive Modulus (MPa) | |
|---|---|---|---|
| PUF | 247.0 | 0.59 | 6.82 |
| PUT | 566.0 | 1.90 | 24.43 |
| Fe-Si/PUC | 340.0 | 0.23 | 1.08 |
| Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Fe-Si (mg/L) | 3.00 | 8.80 | 9.10 | 1.00 | 2.10 | 3.20 | 1.50 |
| Fe (mg/L) | 0.01 | 0.05 | 0.08 | 0.05 | 0.15 | 0.15 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Jamil, S.N.A.M.; Shean Yaw Choong, T.; Abdullah, A.H.; Mastuli, M.S.; Othman, N.; Jiman, N. Green Flexible Polyurethane Foam as a Potent Support for Fe-Si Adsorbent. Polymers 2019, 11, 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11122011
Ahmad A, Jamil SNAM, Shean Yaw Choong T, Abdullah AH, Mastuli MS, Othman N, Jiman N. Green Flexible Polyurethane Foam as a Potent Support for Fe-Si Adsorbent. Polymers. 2019; 11(12):2011. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11122011
Chicago/Turabian StyleAhmad, Afiqah, Siti Nurul Ain Md. Jamil, Thomas Shean Yaw Choong, Abdul Halim Abdullah, Mohd Sufri Mastuli, Nurhanisah Othman, and NurNazurah Jiman. 2019. "Green Flexible Polyurethane Foam as a Potent Support for Fe-Si Adsorbent" Polymers 11, no. 12: 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11122011