Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the Charcoal Precursor
2.3. AC Production
2.4. Characterization of Particle Morphology
2.5. X-ray Diffraction (XRD)
2.6. Thermogravimetric Analysis (TGA)
2.7. Differential Scanning Calorimetry (DSC)
2.8. True Density
2.9. Surface Area and Porosity Structure
2.10. Water Isotherm Measurements
3. Results and Discussion
3.1. Morphological Characteristics
3.2. XRD of the Different Particles
3.3. Thermogravimetry
3.4. Dehydration Heat by DSC and True Density of the Particles
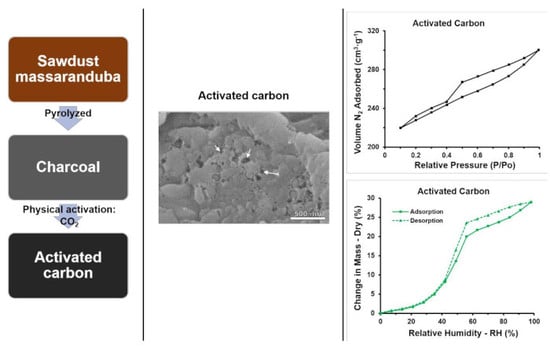
3.5. Surface Area and Porosity Structure
3.6. Water Sorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, H.; Reinoso, F.R. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Avelar, F.F.; Bianchi, M.L.; Gonçalves, M.; da Mota, E.G. The use of piassava fibers (Attalea funifera) in the preparation of activated carbon. Bioresour. Technol. 2010, 101, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Dabioch, M.; Skorek, R.; Kita, A.; Janoska, P.; Pytlakowska, K.; Zerzucha, P.; Sitko, R. A study on adsorption of metals by activated carbon in a large-scale (municipal) process of surface water purification. Cent. Eur. J. Chem. 2013, 11, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.W.; Choi, D.J.; Kim, S.K.; Her, N.; Zoh, K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014, 270, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. BioResources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Structure-mechanics property relationship of waste derived biochars. Sci. Total Environ. 2015, 538, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.G.; Zhang, F.S. Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars. Desalination 2011, 267, 101–106. [Google Scholar] [CrossRef]
- Largitte, L.; Brudey, T.; Tant, T.; Dumesnil, P.C.; Lodewyckx, P. Comparison of the adsorption of lead by activated carbons from three lignocellulosic precursors. Microporous Mesoporous Mater. 2016, 219, 265–275. [Google Scholar] [CrossRef]
- Shaaban, A.; Se, S.M.; Ibrahim, I.M.; Ahsan, Q. Preparation of rubber wood sawdust-based activated carbon and its use as a filler of polyurethane matrix composites for microwave absorption. New Carbon Mater. 2015, 30, 167–175. [Google Scholar] [CrossRef]
- Castro, J.P.; Nobre, J.R.C.; Bianchi, M.L.; Trugilho, P.F.; Napoli, A.; Chiou, B.S.; Williams, T.G.; Wood, D.F.; Avena-Bustillos, R.J.; Orts, W.J.; et al. Activated carbons prepared by physical activation from different pretreatments of Amazon piassava fibers. J. Nat. Fibers 2018. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Ghimbeu, C.M.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. Comptes Rendus Chim. 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Xia, C.; Shi, S.Q. Self-Activation process to fabricate activated carbon from KENAF. Wood Fiber Sci. 2016, 48, 62–69. Available online: https://wfs.swst.org/index.php/wfs/article/view/2342/2271 (accessed on 19 April 2019).
- Jiang, S.; Huang, L.; Nguyen, T.A.H.; Ok, Y.S.; Rudolph, V.; Yang, H.; Zhang, D. Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions. Chemosphere 2016, 142, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Minh, D.P.; Gauthier, D.; Weiss-Hortala, E.; Nzihou, A.; Flamant, G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour. Technol. 2015, 182, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nzihou, A.; Flamant, G.; Stanmore, B. Synthetic fuels from biomass using concentrated solar energy—A review. Energy 2012, 42, 121–131. [Google Scholar] [CrossRef]
- Srinivasakannan, C.; Abu Bakar, M.Z. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.J.; Nimlos, M.R.; Donohoe, B.S. Real-time and post-reaction microscopic structural analysis of biomass undergoing pyrolysis. Energy Fuels 2009, 23, 3810–3817. [Google Scholar] [CrossRef]
- Carraro, P.S.; Spessato, L.; Crespo, L.H.S.; Yokoyama, J.T.C.; Fonseca, J.M.; Bedin, K.C.; Ronix, A.; Cazetta, A.L.; Silva, T.L.; Almeida, V.C. Activated carbon fibers prepared from cellulose and polyester–derived residues and their application on removal of Pb2+ ions from aqueous solution. J. Mol. Liq. 2019, 289. [Google Scholar] [CrossRef]
- Castro, J.B.; Bonelli, P.R.; Cerrella, E.G.; Cukierman, A.L. Phosphoric acid activation of agricultural residues and bagasse from sugar cane: Influence of the experimental conditions on adsorption characteristics of activated carbons. Ind. Eng. Chem. Res. 2000, 39, 4166–4172. [Google Scholar] [CrossRef]
- Mestre, A.S.; Hesse, F.; Freire, C.; Ania, C.O.; Carvalho, A.P. Chemically activated high grade nanoporous carbons from low density renewable biomass (Agave sisalana) for the removal of pharmaceuticals. J. Colloid Interface Sci. 2019, 536, 681–693. [Google Scholar] [CrossRef]
- Gonçalves, M.; Guerreiro, M.C.; Oliveira, L.C.A.; Solar, C.; Nazarro, M.; Sapag, K. Micro Mesoporous Activated Carbon from Coffee Husk as Biomass Waste for Environmental Applications. Waste Biomass Valor. 2013, 4, 395–400. [Google Scholar] [CrossRef]
- Lima, D.R.; Lima, E.C.; Umpierres, C.S.; Thue, P.S.; El-Chaghaby, G.A.; da Silva, R.S.; Pavan, F.A.; Dias, S.L.P.; Biron, C. Removal of amoxicillin from simulated hospital effluents by adsorption using activated carbons prepared from capsules of cashew of Para. Environ. Sci. Pollut. Res. 2019, 26, 16396–16408. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Thummar, M.; Vaghela, M.; Jani, P. Sorption of phenol from aqueous solution using activated carbon prepared from Manilkara zapota seed. Indian J. Chem. Technol. 2008, 15, 533–540. [Google Scholar]
- Freitas, J.V.; Nogueira, F.G.E.; Farinas, C.S. Coconut shell activated carbon as an alternative adsorbent of inhibitors from lignocellulosic biomass pretreatment. Ind. Crop. Prod. 2019, 137, 16–23. [Google Scholar] [CrossRef]
- Tovar, A.K.; Godínez, L.A.; Espejel, F.; Ramírez-Zamora, R.M.; Robles, I. Optimization of the integral valorization process for orange peel waste using a design of experiments approach: Production of high-quality pectin and activated carbon. Waste Manag. 2019, 85, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Nobre, J.R.C.; Castro, J.P.; Motta, J.P.; Bianchi, M.L.; Trugilho, P.F.; Borges, W.M.D.S.; Moulin, J.C. Activated carbon production of the wood residue of the Amazonian area. Sci. For. 2015, 43, 895–906. [Google Scholar] [CrossRef]
- Imazon. A atividade madeireira na Amazônia brasileira: Produção, receitas e mercado. Serviço Florestal Brasileiro (SFB). 2010; p. 20. Available online: http://www.mma.gov.br/estruturas/sfb/_arquivos/atividade_madeireira_outro_pdf_95_1_1_95.pdf (accessed on 13 May 2019).
- Melo, L.E.L.; Silva, C.J.; Lopes, K.V.; Brito, P.G.M.; Santos, I.S. Resíduos de Serraria no Estado do Pará: Caracterização, Quantificação e Utilização Adequada. Floresta Ambient. 2012, 19, 113–116. [Google Scholar] [CrossRef]
- Moulin, J.C.; Nobre, J.R.C.; Castro, J.P.; Trugilho, P.F.; Arantes, M.D.C. Effect of extractives and carbonization temperature on energy characteristics of wood waste in amazon rainforest. Cerne 2017, 23, 209–218. [Google Scholar] [CrossRef]
- Tonoli, G.H.D.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Caixeta, L.A.; Pereira-da-Silva, M.A.; Mattoso, L.H.C. Cellulose micro/nanofibres from Eucalyptus kraft pulp: Preparation and properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef]
- Chan, C.H.; Chia, C.H.; Zakaria, S.; Ahmad, I.; Dufresne, A. Production and characterisation of cellulose and nano-crystalline cellulose from kenaf core wood. BioResources 2013, 8, 785–794. Available online: http://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/BioRes_08_1_Chan_785_Cellulose_Kenaf_Core_Wood/1920 (accessed on 19 April 2019). [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose - structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. Available online: http://www.cellulosechemtechnol.ro/pdf/CCT1-2(2011)/p.13-21.pdf (accessed on 19 April 2019).
- Dunne, L.J.; Manos, G. Perspective and introduction to adsorption and phase behaviour in nanochannels and nanotubes. In Adsorption and Phase Behaviour in Nanochannels and Nanotubes; Dunne, L.J., Manos, G., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–8. [Google Scholar]
- Rashidi, N.A.; Yusup, S.; Hameed, B.H. Kinetic studies on carbon dioxide capture using lignocellulosic based activated carbon. Energy 2013, 61, 440–446. [Google Scholar] [CrossRef]
- Couto, G.M.; Dessimoni, A.L.D.; Bianchi, M.L.; Perigolo, D.M.; Trugilho, P.F. Use of sawdust Eucalyptus sp in the preparation of activated carbons. Ciênc. Agrotec. 2012, 36, 69–77. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Kim, H.J.; Yang, H.S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Órfão, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis kinetics of lignocellulosic materials—three independent reactions model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F. Torrefaction of wood—Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Soares, V.C.; Bianchi, M.L.; Trugilho, P.F.; Höfler, J.; Pereira, A.J. Properties of eucalyptus wood hybrids and charcoal at three ages. Cerne 2015, 21, 191–197. [Google Scholar] [CrossRef]
- Ngernyen, Y.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Porous properties of activated carbon produced from eucalyptus and wattle wood by carbon dioxide activation. Korean J. Chem. Eng. 2006, 23, 1046–1054. [Google Scholar] [CrossRef]
- Su, C.I.; Zeng, Z.L.; Peng, C.C.; Lu, C.H. Effect of temperature and activators on the characteristics of activated carbon fibers prepared from viscose-rayon knitted fabrics. Fibers Polym. 2012, 13, 21–27. [Google Scholar] [CrossRef]
- Hazeleger, M.C.M.; Martinez, J.M.M. Microporosity development by CO2 activation of an anthracite studied by physical adsorption of gases, mercury porosimetry, and scanning electron microscopy. Carbon 1992, 30, 695–709. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yang, R.T. Activated Carbon. In Adsorbents: In Fundamentals and Applications; Yang, R.T., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 79–130. [Google Scholar]
- Brunauer, S. The Adsorption of Gases and Vapors; Oxford University Press: London, UK, 1943. [Google Scholar]
- Boki, K.; Ohno, S. Moisture sorption hysteresis in kudzu starch and sweet-potato starch. J. Food Sci. 1991, 56, 125–127. [Google Scholar] [CrossRef]
- Williams, D.L.; Hodge, D.B. Impacts of delignification and hot water pretreatment on the water induced cell wall swelling behavior of grasses and its relation to cellulolytic enzyme hydrolysis and binding. Cellulose 2014, 21, 221–235. [Google Scholar] [CrossRef]
- Velasco, L.F.; Berezovska, I.; Boutillara, Y.; Lodewyckx, P. The use of organic vapour preadsorption to understand water adsorption on activated carbons. Microporous Mesoporous Mater. 2017, 241, 21–27. [Google Scholar] [CrossRef]
- Velasco, L.F.; Guillet-Nicolas, R.; Dobos, G.; Thommes, M.; Lodewyckx, P. Towards a better understanding of water adsorption hysteresis in activated carbons by scanning isotherms. Carbon 2016, 96, 753–758. [Google Scholar] [CrossRef]
- Lodewyckx, P. The effect of water uptake in ultramicropores on the adsorption of water vapour in activated carbon. Carbon 2010, 48, 2549–2553. [Google Scholar] [CrossRef]
- MacCallum, C.L.; Bandosz, T.J.; McGruder, S.C.; Muller, E.A.; Gubbins, K.E. Molecular model for adsorption of water on activated carbon: Comparison of simulation and experiment. Langmuir 1999, 15, 533–544. [Google Scholar] [CrossRef]
- Muller, E.A.; Hung, F.R.; Gubbins, K.E. Adsorption of water vapor-methane mixtures on activated carbons. Langmuir 2000, 16, 5418–5424. [Google Scholar] [CrossRef]





| Raw Materials | Method and/or Activating Agent Used | Temperature (°C) | S BET a (m2·g−1) | Micropore (cm−3·g−1) | Reference |
|---|---|---|---|---|---|
| Pinewood | CO2 | 800 | 569 | NA b | [7] |
| Guava seeds | CO2:H2O | 850 | 1201 | 0.43 | [8] |
| Wood sawdust | ZnCl2 | 500 | 1301 | 0.37 | [9] |
| Extractive-free piassava | CO2 | 850 | 597 | 0.93 | [10] |
| Olive stones | H2O:N2 | 750 | 807 | 0.30 | [11] |
| Kenaf | Heat and Vacuum | 1100 | 1742 | NA b | [12] |
| Materials a | Tonset (°C) | Heat of Dehydration (J·g−1) | True Density (g.cm−3) |
|---|---|---|---|
| Sawdust | 272 | 174 ± 19 | 1.42 ± 0.00 |
| Charcoal | 457 | 90 ± 15 | 1.44 ± 0.02 |
| Activated carbon (AC) | 531 | 259 ± 46 | 2.64 ± 0.50 |
| Area BET (m2·g−1) | Volume (m3 g−1) | |||
|---|---|---|---|---|
| Macropore | Mesopore | Micropore | Total Pore | |
| 698 | 0.048 | 0.317 | 0.621 | 0.986 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.P.; Nobre, J.R.C.; Napoli, A.; Bianchi, M.L.; Moulin, J.C.; Chiou, B.-S.; Williams, T.G.; Wood, D.F.; Avena-Bustillos, R.J.; Orts, W.J.; et al. Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon. Polymers 2019, 11, 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11081276
Castro JP, Nobre JRC, Napoli A, Bianchi ML, Moulin JC, Chiou B-S, Williams TG, Wood DF, Avena-Bustillos RJ, Orts WJ, et al. Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon. Polymers. 2019; 11(8):1276. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11081276
Chicago/Turabian StyleCastro, Jonnys P., João Rodrigo C. Nobre, Alfredo Napoli, Maria Lucia Bianchi, Jordão C. Moulin, Bor-Sen Chiou, Tina G. Williams, Delilah F. Wood, Roberto J. Avena-Bustillos, William J. Orts, and et al. 2019. "Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon" Polymers 11, no. 8: 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11081276