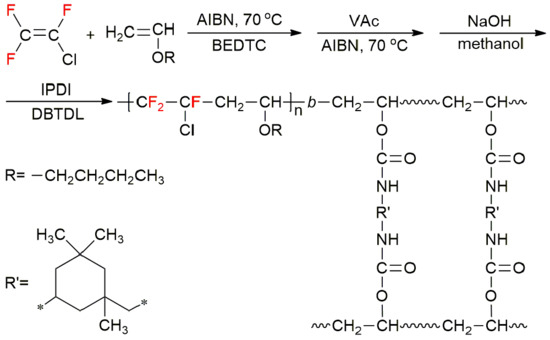
A New Strategy for the Synthesis of Fluorinated Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthetic Procedures
2.3.1. General Procedure for RAFT/MADIX Copolymerization of CTFE and BVE
2.3.2. Synthesis of Poly(CTFE-alt-BVE)-b-PVAc
2.3.3. Synthesis of Poly(CTFE-alt-BVE)-b-PVA
2.3.4. Preparation of the Fluorinated Polyurethane Coating
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wehbi, M.; Banerjee, S.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Vinylidene Fluoride-Based Polymer Network via Cross-Linking of Pendant Triethoxysilane Functionality for Potential Applications in Coatings. Macromolecules 2017, 50, 9329–9339. [Google Scholar] [CrossRef]
- Game, P.; Sage, D.; Chapel, J.P. Surface Mobility of Polyurethane Networks Containing Fluorinated Amphiphilic Reactive Additives. Macromolecules 2002, 35, 917–923. [Google Scholar] [CrossRef]
- McCloskey, C.B.; Yip, C.M.; Santerre, J.P. Effect of Fluorinated Surface-Modifying Macromolecules on the Molecular Surface Structure of a Polyether Poly(urethane urea). Macromolecules 2002, 35, 924–933. [Google Scholar] [CrossRef]
- Wang, L.F. Effect of soft segment length on the thermal behaviors of fluorinated polyurethanes. Eur. Polym. J. 2005, 41, 293–301. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Turri, S.; Valsecchi, R.; Levi, M.; Cristini, M.; Sanguineti, A. Microstructure to property relations in a family of millable polyurethane fluoroelastomers. Eur. Polym. J. 2008, 44, 2951–2961. [Google Scholar] [CrossRef]
- Xu, W.; Lu, B.; Hu, Y.; Song, L.; Nie, S. Synthesis and characterization of novel fluorinated polyurethane elastomers based on 2,2-bis[4-(4-amino-2-trifluoromehyloxyphenyl) phenyl]propane. Polym. Adv. Technol. 2012, 23, 877–883. [Google Scholar] [CrossRef]
- Ge, J.; Si, Y.; Fu, F.; Wang, J.; Yang, J.; Cui, L.; Sun, G. Amphiphobic fluorinated polyurethane composite microfibrous membranes with robust waterproof and breathable performances. RSC Adv. 2013, 3, 2248–2255. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Du, B.; Li, P.; Lai, X.; Niu, Y. Preparation and properties of a novel waterborne fluorinated polyurethane–acrylate hybrid emulsion. Colloid. Polym. Sci. 2014, 292, 579–587. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, F.; Wen, J.; Lv, W.; Porteous, N.; Deng, Y.; Sun, Y. Fluorinated and Un-fluorinated N-halamines as Antimicrobial and Biofilm-controlling Additives for Polymers. Polymer 2015, 68, 92–100. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Yu, J.; Ding, B. Fluorinated polyurethane macroporous membranes with waterproof, breathable and mechanical performance improved by lithium chloride. RSC Adv. 2015, 5, 79807–79814. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, M.; Luo, W.; Yu, J.; Ding, B. Thermally induced chemical cross-linking reinforced fluorinated polyurethane/polyacrylonitrile/polyvinyl butyral nanofibers for waterproof-breathable application. RSC Adv. 2016, 6, 29629–29637. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.L.; Wang, Y.; Qu, D.Z.; Zhang, C.; Li, J.; Bai, Y.P. Synthesis and characterization of fluorinated polyurethane containing carborane in the main chain: thermal, mechanical and chemical resistance properties. Chin. J. Polym. Sci. 2018, 36, 85–97. [Google Scholar] [CrossRef]
- Pali-Casanova, R.; Yam-Cervantes, M.; Zavala-Loría, J.; Loría-Bastarrachea, M.; Aguilar-Vega, M.; Dzul-López, L.; Sámano-Celorio, M.; Crespo-Álvarez, J.; García-Villena, E.; Agudo-Toyos, P.; et al. Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides. Polymers 2019, 11, 1169. [Google Scholar] [CrossRef]
- Rodríguez-Alabanda, Ó.; Romero, P.; Soriano, C.; Sevilla, L.; Guerrero-Vaca, G. Study on the Main Influencing Factors in the Removal Process of Non-Stick Fluoropolymer Coatings Using Nd:YAG Laser. Polymers 2019, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Dong, Q.; Bai, R. Cobalt-Mediated Radical Copolymerization of Chlorotrifluoroethylene and Vinyl Acetate. Polymers 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, X.; Ding, X.; Zheng, Z.; Peng, Y. A novel approach to fluorinated polyurethane by macromonomer copolymerization. Eur. Polym. J. 2005, 41, 1798–1803. [Google Scholar] [CrossRef]
- Chapman, T.M.; Marra, K.G. Determination of Low Critical Surface Tensions of Novel Fluorinated Poly(amide urethane) Block Copolymers. 2. Fluorinated Soft-Block Backbone and Side Chains. Macromolecules 1995, 28, 2081–2085. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, I.; Chun, H.H.; Kim, H.D.; Park, H. Properties of waterborne polyurethane-fluorinated marine coatings: The effect of different types of diisocyanates and tetrafluorobutanediol chain extender content. J. Appl. Polym. Sci. 2014, 131, 39905. [Google Scholar] [CrossRef]
- Wang, S.; Liu, W.; Tan, J. Synthesis and properties of fluorine containing polyurethane based on long chain fluorinated polyacrylate. J. Macromol. Sci. Part. A 2016, 53, 41–48. [Google Scholar] [CrossRef]
- Su, S.-K.; Gu, J.-H.; Lee, H.-T.; Yu, S.-H.; Wu, C.-L.; Suen, M.-C. Effects of an Aromatic Fluoro-Diol and Polycaprolactone on the Properties of the Resultant Polyurethanes. Adv. Polym. Tech. 2018, 37, 1142–1152. [Google Scholar] [CrossRef]
- Takakura, T.; Kato, M.; Yamabe, M. Fluorinated polyurethanes, 1. Synthesis and characterization of fluorine-containing segmented poly(urethane-urea)s. Macromol. Chem. Phys. 1990, 191, 625–632. [Google Scholar] [CrossRef]
- Posada, F.; Gardette, J.L. Photo-oxidation of cured fluorinated polymers IV. Photo-oxidation of the fluorinated copolymer network with urethane linkage. Polym. Degrad. Stab. 2000, 70, 17–29. [Google Scholar] [CrossRef]
- Luda, M.P.; Camino, G.; Laurenti, E.; Novelli, S.; Temtchenko, T.; Turri, S. Mechanism of photostabilization of perfluoropolyether coatings by hindered amine stabilisers. Polym. Degrad. Stab. 2001, 73, 387–392. [Google Scholar] [CrossRef]
- Bassi, M.; Tonelli, C.; Di Meo, A. Glass Transition Behavior of a Microphase Segregated Polyurethane Based on PFPE and IPDI. A Calorimetric Study. Macromolecules 2003, 36, 8015–8023. [Google Scholar] [CrossRef]
- Chen, K.Y.; Kuo, J.F. Influence of fluorocarbon chains on the crystallization behaviors of aliphatic polyurethanes. J. Appl. Polym. Sci. 2009, 111, 371–379. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Wang, Y. Fluorinated waterborne shape memory polyurethane urea for potential medical implant application. J. Appl. Polym. Sci. 2013, 127, 710–716. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Z.; Zhou, C. Study on waterborne polyurethanes based on poly(dimethyl siloxane) and perfluorinated polyether. Macromol. Res. 2015, 23, 867–875. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Li, L.; Liu, Y.; Li, Y.; Dong, Q. Influences of fluorine on microphase separation in fluorinated polyurethanes. Polymer 2016, 98, 311–319. [Google Scholar] [CrossRef]
- Boutevin, B.; Hugon, I.P.; Pietrasanta, Y. Synthese et caracterisation de polyurethanes fluores-II: Étude des polyuréthanes. Eur. Polym. J. 1981, 17, 729–734. [Google Scholar] [CrossRef]
- Boutevin, B.; Hugon, I.P.; Pietrasanta, Y. Synthese et caracterisation de polyurethanes flugres-I: Etude de molecules modeles. Eur. Polym. J. 1981, 17, 723–727. [Google Scholar] [CrossRef]
- Tan, H.; Liu, J.; Li, J.; Jiang, X.; Xie, X.; Zhong, Y.; Fu, Q. Synthesis and Hemocompatibility of Biomembrane Mimicing Poly(carbonate urethane)s Containing Fluorinated Alkyl Phosphatidylcholine Side Groups. Biomacromolecules 2006, 7, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liao, K.H.; Chou, N.K.; Wang, S.S.; Chu, S.H.; Hsieh, K.H. UV-curable low-surface-energy fluorinated poly(urethane-acrylate)s for biomedical applications. Eur. Polym. J. 2008, 44, 2937. [Google Scholar] [CrossRef]
- Shevchenko, V.V.; Sidorenko, A.V.; Bliznyuk, V.N.; Tkachenko, I.M.; Shekera, O.V.; Smirnov, N.N.; Tsukruk, V.V. Synthesis and properties of hydroxylated core-fluorinated diamines and polyurethanes based on them with azobenzene nonlinear optical chromophores in the backbone. Polymer 2013, 54, 6516–6525. [Google Scholar] [CrossRef]
- Wu, C.L.; Chiu, S.H.; Lee, H.T.; Suen, M.C. Synthesis and properties of biodegradable polycaprolactone/polyurethanes using fluoro chain extenders. Polym. Adv. Technol. 2016, 27, 665–676. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Peng, X. Synthesis and characterization of novel fluorinated thermoplastic polyurethane with high transmittance and superior physical properties. J. Macromol. Sci. Part. A 2017, 54, 516–523. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.; Wang, Y.; Qu, D.; Hu, W.; Luan, Y.; Dong, S.; Zhang, J.; Bai, Y. Fluorinated polyurethane based on liquid fluorine elastomer (LFH) synthesis via two-step method: the critical value of thermal resistance and mechanical properties. RSC Adv. 2017, 7, 30970–30978. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Zhang, X.; Dai, J.; Li, W.; Luo, Y. Study of Surface Properties of Novel Fluorinated Polyurethanes with Fluorine-Containing Pendent Groups. J. Macromol. Sci. Part. A 2009, 46, 215–221. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, X.; Dai, J.; Li, W.; Luo, Y. Synthesis, characterization and properties of a novel fluorinated polyurethane. Eur. Polym. J. 2009, 45, 530–536. [Google Scholar] [CrossRef]
- Ho, T.; Wynne, K.J. A new fluorinated polyurethane polymerization, characterization, and mechanical properties. Macromolecules 1992, 25, 3521–3527. [Google Scholar] [CrossRef]
- Liu, P.; Ye, L.; Liu, Y.; Nie, F. Preparation and properties of the main-chain-fluorinated thermoplastic polyurethane elastomer. Polym. Bull. 2010, 66, 503–515. [Google Scholar] [CrossRef]
- Fournier, D.; Du Prez, F. “Click” Chemistry as a Promising Tool for Side-Chain Functionalization of Polyurethanes. Macromolecules 2008, 41, 4622–4630. [Google Scholar] [CrossRef]
- Zhu, M.J.; Qing, F.L.; Meng, W.D. Novel waterborne polyurethanes containing short fluoroalkyl chains: Synthesis and applications on cotton fabrics. J. Appl. Polym. Sci. 2008, 109, 1911–1915. [Google Scholar] [CrossRef]
- Pilch-Pitera, B. Blocked polyisocyanates containing fluorine atoms as crosslinking agents for polyurethane powder coatings. J. Appl. Polym. Sci. 2012, 124, 3302–3311. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, Z.; Zhang, Y.; Tuo, X. Synthesis and simultaneous self-assembly of multiblock fluorinated polyurethane in iniferter polymerization. J. Polym. Sci. Part. A Polym. Chem. 2013, 51, 2161–2170. [Google Scholar] [CrossRef]
- Yang, W.; Pan, P.; Wang, H.; Cheng, X.; Du, Z. Effect of hydroxyl-terminated poly(fluoroalkyl methacrylate) main-chain length on the microphase separation of waterborne fluorinated polyurethane. Polym. Int. 2018, 67, 1054–1061. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Li, J.; Tan, H.; Zhong, Y.; Fu, Q. Surface and bulk properties of poly(ether urethane)s/fluorinated phosphatidylcholine polyurethanes blends. J. Appl. Polym. Sci. 2008, 108, 548–553. [Google Scholar] [CrossRef]
- Jiang, G.; Tuo, X.; Wang, D.; Li, Q. Preparation, characterization, and properties of fluorinated polyurethanes. J. Polym. Sci., Part. A Polym. Chem. 2009, 47, 3248–3256. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, Y.H.; Park, H.; Kim, H.D. Preparation and properties of UV-curable fluorinated polyurethane acrylates. J. Appl. Polym. Sci. 2014, 131, 40603. [Google Scholar] [CrossRef]
- Jeon, J.H.; Park, Y.G.; Lee, Y.H.; Lee, D.J.; Kim, H.D. Preparation and properties of UV-curable fluorinated polyurethane acrylates containing crosslinkable vinyl methacrylate for antifouling coatings. J. Appl. Polym. Sci. 2015, 132, 42168. [Google Scholar] [CrossRef]
- Jia, R.P.; Zong, A.X.; He, X.Y.; Xu, J.Y.; Huang, M.S. Synthesis of newly fluorinated thermoplastic polyurethane elastomers and their blood compatibility. Fibers Polym. 2015, 16, 231–238. [Google Scholar] [CrossRef]
- Park, J.M.; Jeon, J.H.; Lee, Y.H.; Lee, D.J.; Park, H.; Chun, H.H.; Do Kim, H. Synthesis and properties of UV-curable polyurethane acrylates containing fluorinated acrylic monomer/vinyltrimethoxysilane. Polym. Bull. 2015, 72, 1921–1936. [Google Scholar] [CrossRef]
- Su, S.K.; Gu, J.H.; Lee, H.T.; Wu, C.L.; Hwang, J.J.; Suen, M.C. Synthesis and properties of novel biodegradable polyurethanes containing fluorinated aliphatic side chains. J. Polym. Res. 2017, 24, 142. [Google Scholar] [CrossRef]
- Shi, X.; Shi, H.; Wu, H.; Shen, H.; Cao, P. Synthesis and properties of novel fluorinated polyurethane based on fluorinated gemini diol. Polym. Adv. Technol. 2018, 29, 1939–1952. [Google Scholar] [CrossRef]
- Tan, J.; Liu, W.; Wang, Z. Preparation and performance of waterborne UV-curable polyurethane containing long fluorinated side chains. J. Appl. Polym. Sci. 2017, 134, 44506. [Google Scholar] [CrossRef]
- Chen, M.; Ou, B.; Guo, Y.; Guo, Y.; Kang, Y.; Liu, H.; Yan, J.; Tian, L. Preparation of an environmentally friendly antifouling degradable polyurethane coating material based on medium-length fluorinated diols. J. Macromol. Sci. Part. A 2018, 55, 483–488. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Zong, J.; Zhang, S.; Deng, Q.; Liu, S. Synthesis of a fluoro-diol and preparation of fluorinated waterborne polyurethanes with high elongation at break. J. Macromol. Sci. Part. A 2017, 55, 183–191. [Google Scholar] [CrossRef]
- Chapman, T.M.; Benrashid, R.; Gribbin, K.L.; Keener, J.P. Determination of Low Critical Surface Energies of Novel Fluorinated Poly(amide urethane) Block Copolymers. 1. Fluorinated Side Chains. Macromolecules 1995, 28, 331–335. [Google Scholar] [CrossRef]
- Tan, D.S.; Zhang, X.Q.; Wang, J.C.; Li, J.H.; Tan, H.; Fu, Q. Synthesis and phase behavior of polyurethanes end-capped with fluorinated phosphatidylcholine head groups. Chin. J. Polym. Sci. 2011, 29, 615–626. [Google Scholar] [CrossRef]
- Zhu, Q.; Han, C.C. Study of telechelic polyurethane with perfluoropolyether tails. Polymer 2010, 51, 877–882. [Google Scholar] [CrossRef]
- Penoff, M.; Schreiner, W.; Oyanguren, P.; Montemartini, P. Fluorinated Polyurethanes: XPS and AFM Characterization. Macromol. Symp. 2012, 321, 186–190. [Google Scholar] [CrossRef]
- Mao, X.; Chen, Y.; Si, Y.; Li, Y.; Wan, H.; Yu, J.; Ding, B. Novel fluorinated polyurethane decorated electrospun silica nanofibrous membranes exhibiting robust waterproof and breathable performances. RSC Adv. 2013, 3, 7562–7569. [Google Scholar] [CrossRef]
- Gennen, S.; Grignard, B.; Thomassin, J.M.; Gilbert, B.; Vertruyen, B.; Jerome, C.; Detrembleur, C. Polyhydroxyurethane hydrogels: Synthesis and characterizations. Eur. Polym. J. 2016, 84, 849–862. [Google Scholar] [CrossRef]
- Li, P.; Shen, Y.; Yang, X.; Li, G. Preparation and properties of waterborne cationic fluorinated polyurethane. J. Polym. Res. 2012, 19, 9786. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Lyakhova, K.; van der Ven, L.G.J.; van Benthem, R.A.T.M.; de With, G. Surface Segregation of Low Surface Energy Polymeric Dangling Chains in a Cross-Linked Polymer Network Investigated by a Combined Experimental–Simulation Approach. Macromolecules 2013, 46, 1993–2002. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, P.; Wang, C.; Luo, Q. Study on properties of waterborne fluorinated polyurethane/acrylate hybrid emulsion and films. J. Polym. Res. 2014, 21, 460. [Google Scholar] [CrossRef]
- Wayland, B.B.; Poszmik, G.; Mukerjee, S.L.; Fryd, M. Living radical polymerization of acrylates by organocobalt porphyrin complexes. J. Am. Chem. Soc. 1994, 116, 7943–7944. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process-a second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tonnar, J.; Ameduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of Iodocompounds in Radical Polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Ameduri, B.; Hung, M.H. Telechelic Diiodopoly(VDF-co-PMVE) Copolymers by Iodine Transfer Copolymerization of Vinylidene Fluoride (VDF) with Perfluoromethyl Vinyl Ether (PMVE)†. Macromolecules 2010, 43, 3652–3663. [Google Scholar] [CrossRef]
- Sawada, H.; Tashima, T.; Nishiyama, Y.; Kikuchi, M.; Goto, Y.; Kostov, G.; Ameduri, B. Iodine Transfer Terpolymerization of Vinylidene Fluoride, α-Trifluoromethacrylic Acid and Hexafluoropropylene for Exceptional Thermostable Fluoropolymers/Silica Nanocomposites. Macromolecules 2011, 44, 1114–1124. [Google Scholar] [CrossRef]
- Kyulavska, M.; Kostov, G.; Ameduri, B.; Mateva, R. Unexpected alternating radical copolymerization of chlorotrifluoroethylene with 3-isopropenyl-α, α′-dimethylbenzyl isocyanate. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 2681–2697. [Google Scholar] [CrossRef]
- Liu, L.; Lu, D.; Wang, H.; Dong, Q.; Wang, P.; Bai, R. Living/controlled free radical copolymerization of chlorotrifluoroethene and butyl vinyl ether under 60Co γ-ray irradiation in the presence of S-benzyl O-ethyl dithiocarbonate. Chem. Commun. 2011, 47, 7839–7841. [Google Scholar] [CrossRef] [PubMed]
- Girard, E.; Marty, J.-D.; Ameduri, B.; Destarac, M. Direct Synthesis of Vinylidene Fluoride-Based Amphiphilic Diblock Copolymers by RAFT/MADIX Polymerization. ACS Macro Lett. 2012, 1, 270–274. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.; Liu, L.; Jin, B.; Bai, R. Xanthate-mediated living/controlled radical copolymerization of hexafluoropropylene and butyl vinyl ether under 60Co γ-ray irradiation and preparation of fluorinated polymers end-capped with a fluoroalkyl sulfonic acid group. Polym. Chem. 2013, 4, 1760–1764. [Google Scholar] [CrossRef]
- Wang, P.; Dai, J.; Liu, L.; Dong, Q.; Wang, H.; Bai, R. Synthesis and properties of a well-defined copolymer of chlorotrifluoroethylene and N-vinylpyrrolidone by xanthate-mediated radical copolymerization under 60 Co γ-ray irradiation. Polym. Chem. 2014, 5, 6358–6364. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Organometallic-Mediated Radical Polymerization of Vinylidene Fluoride. Angew. Chem. Int. Ed. Engl. 2018, 57, 2934–2937. [Google Scholar] [CrossRef]
- Kostov, G.; Boschet, F.; Buller, J.; Badache, L.; Brandsadter, S.; Ameduri, B. First amphiphilic poly (vinylidene fluoride-co-3, 3, 3-trifluoropropene)-b-oligo (vinyl alcohol) block copolymers as potential nonpersistent fluorosurfactants from radical polymerization controlled by xanthate. Macromolecules 2011, 44, 1841–1855. [Google Scholar] [CrossRef]
- Guerre, M.; Uchiyama, M.; Lopez, G.; Améduri, B.; Satoh, K.; Kamigaito, M.; Ladmiral, V. Synthesis of PEVE-b-P(CTFE-alt-EVE) block copolymers by sequential cationic and radical RAFT polymerization. Polym. Chem. 2018, 9, 352–361. [Google Scholar] [CrossRef]
- Pound, G.; McLeary, J.B.; McKenzie, J.M.; Lange, R.F.; Klumperman, B. In-situ NMR spectroscopy for probing the efficiency of RAFT/MADIX agents. Macromolecules 2006, 39, 7796–7797. [Google Scholar] [CrossRef]
- Carnevale, D.; Wormald, P.; Ameduri, B.; Tayouo, R.; Ashbrook, S.E. Multinuclear Magnetic Resonance and DFT Studies of the Poly(chlorotrifluoroethylene-alt-ethyl vinyl ether) Copolymers. Macromolecules 2009, 42, 5652–5659. [Google Scholar] [CrossRef]
- Lu, Y.; Claude, J.; Zhang, Q.; Wang, Q. Microstructures and Dielectric Properties of the Ferroelectric Fluoropolymers Synthesized via Reductive Dechlorination of Poly(vinylidene fluoride-co-chlorotrifluoroethylene)s. Macromolecules 2006, 39, 6962–6968. [Google Scholar] [CrossRef]
- Tiers, G.V.D.; Bovey, F.A. Polymer NMR spectroscopy. VII. The stereochemical configuration of polytrifluorochloroethylene. J. Polym. Sci. Part. A General Papers 1963, 1, 833–841. [Google Scholar] [CrossRef]









| Sample | Time/h | Conv.1 | Mn,th | Mn,NMR | Mn,GPC | Mw/Mn |
|---|---|---|---|---|---|---|
| P1 | 1 | 0.11 | 2500 | 2400 | 4600 | 1.41 |
| P2 | 2 | 0.22 | 5000 | 5200 | 6900 | 1.38 |
| P3 | 3 | 0.31 | 6900 | 7300 | 8900 | 1.43 |
| P4 | 4 | 0.40 | 8900 | 8500 | 10,900 | 1.37 |
| P5 | 5 | 0.50 | 11,000 | 11,000 | 13,000 | 1.37 |
| P6 | 6 | 0.56 | 12,300 | 12,200 | 14,500 | 1.35 |
| P7 | 9 | 0.66 | 14,500 | 14,800 | 16,800 | 1.39 |
| Sample 1 | Solvent | |||||||
|---|---|---|---|---|---|---|---|---|
| Water | H2SO4 (10 wt%) | NaOH (10 wt%) | Ethyl Acetate | Acetone | Ethanol | CHCl3 | THF | |
| FPU | 10 | 10 | 10 | 8 | 8 | 10 | 8 | 8 |
| PCB | 10 | 10 | 10 | 0 | 0 | 10 | 2 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-C.; Lu, D.; Wang, H.; Bai, R.-K. A New Strategy for the Synthesis of Fluorinated Polyurethane. Polymers 2019, 11, 1440. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091440
Wang P-C, Lu D, Wang H, Bai R-K. A New Strategy for the Synthesis of Fluorinated Polyurethane. Polymers. 2019; 11(9):1440. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091440
Chicago/Turabian StyleWang, Pu-Cheng, Dan Lu, Hu Wang, and Ru-Ke Bai. 2019. "A New Strategy for the Synthesis of Fluorinated Polyurethane" Polymers 11, no. 9: 1440. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11091440