Kinetic Studies on the Catalytic Degradation of Rhodamine B by Hydrogen Peroxide: Effect of Surfactant Coated and Non-Coated Iron (III) Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis and Surfactant Coating of Fe3O4 Magnetic NPs
2.3. Characterization
2.4. Degradation Kinetic Experiments
3. Results and Discussion
3.1. Characterization of Fe3O4 and SDS@Fe3O4 NPs
3.1.1. X-ray Diffraction (XRD)
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Transmission Electron Microscopy (TEM)
3.1.5. Vibrating Sample Magnetometer (VSM)
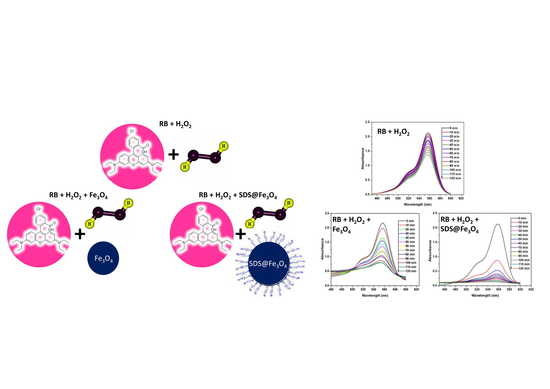
3.2. Degradation of RB by H2O2
3.3. Degradation of RB in the Presence of Fe3O4 and SDS@Fe3O4 NPs
- (i)
- The possible reactions of free radicals are:
- (ii)
- Fe2+,
- (iii)
- H2O2 H2O HO2*,
- (iv)
- H2O O2,
- (v)
- H2O2,
- (vi)
- + RBProducts,
- (vii)
- + RB Products.
3.4. Effect of SDS Concentration and Fe3O4 NPs Dosage on RB Degradation
3.5. Effect of Temperature on RB Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Refaie, K.; Ghfar, A.A.; Wabaidur, S.M.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A.; Alqadami, A.A.; Hamid, M. Cetyltrimethylammonium bromide intercalated and branched polyhydroxystyrene functionalized montmorillonite clay to sequester cationic dyes. J. Environ. Manag. 2018, 219, 285–293. [Google Scholar]
- Khan, M.A.; Wabaidur, S.M.; Siddiqui, M.R.; Alqadami, A.A.; Khan, A.H. Silico-Manganese Fumes Waste Encapsulated Cryogenic Alginate Beads for Aqueous Environment De-colorization. J. Clean. Prod. 2020, 244, 118867. [Google Scholar] [CrossRef]
- Goyal, P.; Chakraborty, S.; Misra, S.K. Multifunctional Fe3O4-ZnO nanocomposites for environmental remediation applications. Environ. Nanotechnol. Monit. Manag. 2018, 10, 28–35. [Google Scholar] [CrossRef]
- Khan, M.A.; Siddiqui, M.R.; Otero, M.; Alshareef, S.A.; Rafatullah, M. Removal of rhodamine B from water using a solvent impregnated polymeric Dowex 5WX8 resin: Statistical optimization and batch adsorption studies. Polymers 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Ranjbari, E.; Hadjmohammadi, M.R.; Kiekens, F.; De Wael, K. Mixed Hemi/Ad-Micelle Sodium Dodecyl Sulfate-Coated Magnetic Iron Oxide Nanoparticles for the Efficient Removal and Trace Determination of Rhodamine-B and Rhodamine-6G. Anal. Chem. 2015, 87, 7894–7901. [Google Scholar] [CrossRef]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Ngo, T.M.V.; Truong, T.H.; Nguyen, T.H.L.; Duong, T.T.A.; Vu, T.H.; Pham, T.D. Surface modified laterite soil with an anionic surfactant for the removal of a cationic dye (crystal violet) from an aqueous solution. Water Air Soil Pollut. 2020, 231, 1–15. [Google Scholar] [CrossRef]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, characterization, and modification of alumina nanoparticles for cationic dye removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Zhang, T.W.; Wilhelm, P.; Stephan, D.; Carp, O.; Huisman, C.L.; Reller, A.; Topare, N.S.; Bisen, N.; Shrivastava, P.; et al. Photocatalytic Degradation of Rhodamine B by using UV/TiO2 and Nb2O5 Process: A Kinetic Study. J. Photochem. Photobiol. A Chem. 2004, 162, 33–177. [Google Scholar] [CrossRef]
- Yan, P.; Gao, L.B.; Li, W.T. Microwave-enhanced fenton-like system, Fe3O4/H2O2, for Rhodamine B wastewater degradation. Appl. Mech. Mater. 2013, 448, 834–837. [Google Scholar] [CrossRef]
- Mehrdad, A.; Hashemzadeh, R. Ultrasonic degradation of Rhodamine B in the presence of hydrogen peroxide and some metal oxide. Ultrason. Sonochem. 2010, 17, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite nanoparticles for removal of heavy metals from aqueous solutions: Synthesis and characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Dalali, N.; Khoramnezhad, M.; Habibizadeh, M.; Faraji, M. Magnetic Removal of Acidic Dyes from Waste Waters Using Surfactant- Coated Magnetite Nanoparticles: Optimization of Process by Taguchi Method. In Proceedings of the 2011 International Conference on Environmental and Agriculture Engineering IPCBEE, Chengdu, China, 29–31 July 2011; pp. 89–93. [Google Scholar]
- Jiaqi, Z.; Yimin, D.; Danyang, L.; Shengyun, W.; Liling, Z.; Yi, Z. Synthesis of carboxyl-functionalized magnetic nanoparticle for the removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 58–66. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Alishiri, T.; Oskooei, H.A.; Heravi, M.M. Fe 3 O 4 Nanoparticles as an Efficient and Magnetically Recoverable Catalyst for the Synthesis of α,β-Unsaturated Heterocyclic and Cyclic Ketones under Solvent-Free Conditions. Synth. Commun. 2013, 43, 3357–3362. [Google Scholar] [CrossRef]
- Godoi, M.; Liz, D.G.; Ricardo, E.W.; Rocha, M.S.T.; Azeredo, J.B.; Braga, A.L. Magnetite (Fe3O4) nanoparticles: An efficient and recoverable catalyst for the synthesis of alkynyl chalcogenides (selenides and tellurides) from terminal acetylenes and diorganyl dichalcogenides. Tetrahedron 2014, 70, 3349–3354. [Google Scholar] [CrossRef]
- Wu, X.; Nan, Z. Degradation of rhodamine B by a novel Fe3O4 /SiO2 double-mesoporous-shelled hollow spheres through photo-Fenton process. Mater. Chem. Phys. 2019, 227, 302–312. [Google Scholar] [CrossRef]
- Jiao, Y.; Wan, C.; Bao, W.; Gao, H.; Liang, D.; Li, J. Facile hydrothermal synthesis of Fe3O4@cellulose aerogel nanocomposite and its application in Fenton-like degradation of Rhodamine B. Carbohydr. Polym. 2018, 189, 371–378. [Google Scholar] [CrossRef]
- AlHamedi, F.H.; Rauf, M.A.; Ashraf, S.S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, A.A.; Yamini, Y.; Faraji, M.; Nourmohammadian, F. Modified magnetite nanoparticles with cetyltrimethylammonium bromide as superior adsorbent for rapid removal of the disperse dyes from wastewater of textile companies. Nanochem. Res. 2016, 1, 49–56. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.T.L.; Pham, T.D.; Le, T.S. Removal of lindane from aqueous solution using aluminum hydroxide nanoparticles with surface modification by anionic surfactant. Polymers 2020, 12, 960. [Google Scholar] [CrossRef]
- Sun, C.; Yang, S.T.; Gao, Z.; Yang, S.; Yilihamu, A.; Ma, Q.; Zhao, R.S.; Xue, F. Fe3O4 /TiO2 /reduced graphene oxide composites as highly efficient Fenton-like catalyst for the decoloration of methylene blue. Mater. Chem. Phys. 2019, 223, 751–757. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Wang, F.H.; Kehr, J.; Bjelke, B.; Zhang, Y.; Tsakalakos, T.; Muhammed, M. Starch-Coated Superparamagnetic Nanoparticles as MR Contrast Agents. Chem. Mater. 2003, 15, 4343–4351. [Google Scholar] [CrossRef]
- Zhang, J.L.; Srivastava, R.S.; Misra, R.D.K. Core−Shell Magnetite Nanoparticles Surface Encapsulated with Smart Stimuli-Responsive Polymer: Synthesis, Characterization, and LCST of Viable Drug-Targeting Delivery System. Langmuir 2007, 23, 6342–6351. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI-PEG-Chitosan-Copolymer-Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, Complexation, and Transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef] [Green Version]
- El-kharrag, R.; Amin, A.; Greish, Y.E. Synthesis and characterization of mesoporous sodium dodecyl sulfate-coated magnetite nanoparticles. J. Ceram. Sci. Technol. 2011, 2, 203–210. [Google Scholar] [CrossRef]
- Abedi, M.H.; Ahmadmoazzam, M.; Jaafarzadeh, N. Removal of cationic tolonium chloride dye using Fe3O4 nanoparticles modified with sodium dodecyl sulfate. Chem. Biochem. Eng. Q. 2018, 32, 205–213. [Google Scholar] [CrossRef]
- Yamini, Y.; Faraji, M.; Rajabi, A.A.; Nourmohammadian, F. Ultra efficient removal of Basic Blue 41 from textile industry’s wastewaters by sodium dodecyl sulphate coated magnetite nanoparticles: Removal, kinetic and isotherm study. Anal. Bioanal. Chem. Res. 2018, 5, 205–215. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Feshki, S. Solid-phase extraction and separation of brilliant green by Fe3O4 magnetic nano-particles functionalized by sodium dodecyl sulphate from aqueous solution: Multivariate optimization and adsorption characterization. Desalin. Water Treat. 2017, 75, 58–69. [Google Scholar] [CrossRef]
- Raees, K.; Ansari, M.S.; Rafiquee, M.Z.A. Influence of surfactants and surfactant-coated IONs on the rate of alkaline hydrolysis of procaine in the presence of PEG. J. King Saud Univ. Sci. 2020, 32, 1182–1189. [Google Scholar] [CrossRef]
- Raees, K.; Ansari, M.S.; Rafiquee, M.Z.A. Inhibitive effect of super paramagnetic iron oxide nanoparticles on the alkaline hydrolysis of procaine. J. Nanostruct. Chem. 2019, 9, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Jeon, B.-H.; Alshareef, S.A.; Alothman, Z.A.; Hamedelniel, A.E. Oil industry waste based non-magnetic and magnetic hydrochar to sequester potentially toxic post-transition metal ions from water. J. Hazard. Mater. 2020, 400, 123247. [Google Scholar] [CrossRef]
- Saranya, T.; Parasuraman, K.; Anbarasu, M.; Balamurugan, K. XRD, FT-IR and SEM Study of Magnetite (Fe3O4) Nanoparticles Prepared by Hydrothermal Method. Nano Vis. 2015, 5, 149–154. [Google Scholar]
- Aliramaji, S.; Zamanian, A.; Sohrabijam, Z. Characterization and Synthesis of Magnetite Nanoparticles by Innovative Sonochemical Method. Procedia Mater. Sci. 2015, 11, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Saif, B.; Wang, C.; Chuan, D.; Shuang, S. Synthesis and Characterization of Fe3O4 Coated on APTES as Carriers for Morin-Anticancer Drug. J. Biomater. Nanobiotechnol. 2015, 6, 267–275. [Google Scholar] [CrossRef]
- Azari, Z.; Pourbasheer, E.; Beheshti, A. Mixed hemimicelles solid-phase extraction based on sodium dodecyl sulfate (SDS)-coated nano-magnets for the spectrophotometric determination of Fingolomid in biological fluids, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 599–604. [Google Scholar] [CrossRef]
- Tabrizi, A.B.; Teymurlouie, N.D. Application of Sodium Dodecyl Sulfate Coated Iron Oxide Magnetic Nanoparticles for the Extraction and Spectrofluorimetric Determination of Propranolol in Different Biological Samples. J. Mex. Chem. Soc. 2018, 60, 108–116. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.-H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Nacéra, Z. Comparative study of discoloration of mono-azo dye by catalytic oxidation based on wells-dawson heteropolyanion catalyst. Environ. Nanotechnol. Monit. Manag. 2018, 10, 10–16. [Google Scholar] [CrossRef]
- Wang, B.; Yin, J.-J.; Zhou, X.; Kurash, I.; Chai, Z.; Zhao, Y.; Feng, W. Physicochemical Origin for Free Radical Generation of Iron Oxide Nanoparticles in Biomicroenvironment: Catalytic Activities Mediated by Surface Chemical States. J. Phys. Chem. C 2013, 117, 383–392. [Google Scholar] [CrossRef]
- Jiang, J.; Zou, J.; Zhu, L.; Huang, L.; Jiang, H.; Zhang, Y. Degradation of methylene blue with H2O2 activated by peroxidase-like Fe3O4 magnetic nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Peres, J. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dye Pigment 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Ma, Y.S.; Chang, C.N.; Chao, C.R. Decolorization of Rhodamine B by a Photo-Fenton Process: Effect of System Parameters and Kinetic Study. Int. J. Environ. Resour. 2012, 1, 73–80. [Google Scholar]
- Gulkaya, I.; Surucu, G.; Dilek, F. Importance of H2O2/Fe2+ ratio in Fenton’s treatment of a carpet dyeing wastewater. J. Hazard. Mater. 2006, 136, 763–769. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.; Gulshan, F. Photocatalytic Degradation of Methylene Blue by Magnetite+H2O2+UV Process. Int. J. Environ. Sci. Dev. 2016, 7, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Keyhanian, F.; Shariati, S.; Faraji, M.; Hesabi, M. Magnetite nanoparticles with surface modification for removal of methyl violet from aqueous solutions. Arab. J. Chem. 2016, 9, S348–S354. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Rather, M.A.; Bhat, S.A.; Pandit, S.A.; Bhat, F.A.; Rather, G.M.; Bhat, M.A. As Catalytic as Silver Nanoparticles Anchored to Reduced Graphene Oxide: Fascinating Activity of Imidazolium Based Surface Active Ionic Liquid for Chemical Degradation of Rhodamine B. Catal. Lett. 2019, 149, 2195. [Google Scholar] [CrossRef]
- Khan, A.M.; Mehmood, A.; Sayed, M.; Nazar, M.F.; Ismail, B.; Khan, R.A.; Ullah, H.; Rehman, H.M.A.; Khan, A.Y.; Khan, A.R. Influence of acids, bases and surfactants on the photocatalytic degradation of a model dye rhodamine B. J. Mol. Liq. 2017, 236, 395–403. [Google Scholar] [CrossRef]
- Oliveira, E.G.L.; Rodrigues, J.J.; de Oliveira, H.P. Influence of surfactant on the fast photodegradation of rhodamine B induced by TiO2 dispersions in aqueous solution. Chem. Eng. J. 2011, 172, 96–101. [Google Scholar] [CrossRef]











| Reaction Media | Ea (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1K−1) | R2 |
|---|---|---|---|---|
| H2O2 | 69.47 | 66.85 | –132.66 | 0.949 |
| H2O2 + Fe3O4 | 28.47 | 25.86 | –188.18 | 0.953 |
| H2O2 + Fe3O4 + SDS | 32.47 | 29.86 | –194.42 | 0.945 |
| H2O2 + SDS@Fe3O4 | 15.63 | 13.01 | –149.00 | 0.953 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.S.; Raees, K.; Ali Khan, M.; Rafiquee, M.Z.A.; Otero, M. Kinetic Studies on the Catalytic Degradation of Rhodamine B by Hydrogen Peroxide: Effect of Surfactant Coated and Non-Coated Iron (III) Oxide Nanoparticles. Polymers 2020, 12, 2246. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102246
Ansari MS, Raees K, Ali Khan M, Rafiquee MZA, Otero M. Kinetic Studies on the Catalytic Degradation of Rhodamine B by Hydrogen Peroxide: Effect of Surfactant Coated and Non-Coated Iron (III) Oxide Nanoparticles. Polymers. 2020; 12(10):2246. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102246
Chicago/Turabian StyleAnsari, Mohd Shaban, Kashif Raees, Moonis Ali Khan, M.Z.A. Rafiquee, and Marta Otero. 2020. "Kinetic Studies on the Catalytic Degradation of Rhodamine B by Hydrogen Peroxide: Effect of Surfactant Coated and Non-Coated Iron (III) Oxide Nanoparticles" Polymers 12, no. 10: 2246. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12102246