Microwave Foaming of Materials: An Emerging Field
Abstract
:1. Introduction
2. Principle, Mechanism, and Steps in the Microwave Foaming
2.1. Starch-Based Foams
2.2. Polystyrene and Syntactic Foams
2.3. Phenolic Foams
2.4. Modified Microwave Foaming Processes
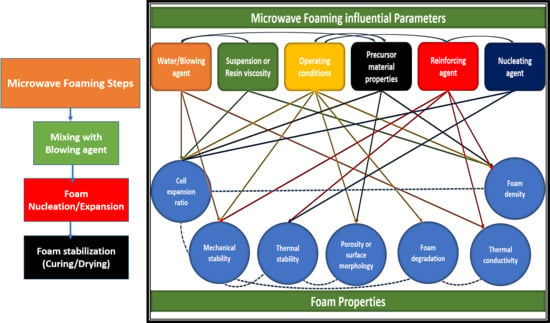
3. Effect of Microwave Foaming Influential Factors on the Final Properties of Foamed Materials
3.1. Effect of Water or Blowing Agent Content
3.2. Effect of Suspension/Resin Viscosity
3.3. Effect of Microwave Operating Variables
3.4. Effect of Foam Precursor Materials Properties
3.5. Effect of Reinforcing Agents
3.6. Effect of Additives/Nucleating Agents
4. Discussion: Towards the Development of Industrial-Scale Microwave Foaming Processes
5. Conclusions and Future Recommendations
- Utilization of new or sustainable higher dielectric loss blowing agents, e.g., water or their new combinations, to achieve better dispersion and foaming of different matrix materials that are incredibly transparent to microwaves. This is expected to provide effective heating and bubble growth and resulted in low-density cellular foams while overcoming the reliance on less sustainable blowing agents such as HCFCs and pentane.
- Dielectric characterization of foaming materials, additives, and blowing agents is highly desirable to understand better the microwave interaction with these materials, the importance of foaming temperature, and the overall foaming process. This will underpin not only the formulation of matrix-blowing agent-filler systems but also the design of bespoke microwave heating applicators and scale-up to industrial production.
- Rheological measurements such as viscosity, melt strength, and elongation strength are also vital issues required for providing an in-depth understanding of ways for controlling foaming, bubble stabilization, collapse, and expansion ratios for the production of foams with the desired requirements. Coupling this understanding with dielectric characterization also provides an opportunity to refine microwave heating protocols further.
- Use of nanofillers/modified fillers for both reinforcement and as nucleating agents for facilitating the foaming process in stabilizing bubbles growth. This is expected to result in higher expansion, low density, and thermally and stable mechanical foams.
- Modified microwave foaming techniques such as chemical-assisted microwave foaming in helping to induce cross-linking, branching, or degradation before foaming to achieve better foaming reduces the risk of cell collapse, improved expansion, and lower density cellular morphology of product, which has thus far proved challenging in some microwave foaming applications
- Development of new composite structures, e.g., syntactic polymer composites which cannot be readily produced using conventional foaming technologies
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EPS | Expanded polystyrene |
| EPE | expanded polyethylene |
| EPU | Expanded Polyurethane |
| PLA | Poly(lactic acid) |
| MC | Moisture content |
| AC | Activated carbon |
| MWCNT | Multi-wall carbon nanotubes |
| EI | Expansion index |
| PTSA | p-toluenesulfonic acid |
| BA | Bottom ash |
| ADC | Azodicarbonamide |
| TPUR | Thermoplastic polyurethanes |
| CB | Carbon black |
| OPEX | Operating expense |
| CAPEX | Capital expense |
| PVOH | Polyvinyl alcohol |
| Sodium chloride | NaCl |
| Calcium chloride | CaCl2 |
| BIH | Hydrocerol |
| MC | Moisture content |
| SS | Sodium silicate |
| PEGDA | Polyethylene glycol diacrylate |
| CS | Chitosan |
| Ti | Titanium |
| Al | Aluminum |
| BC | Boron carbide |
| PCL | Poly(caprolactone) |
| DCM | Dichloromethane |
| BPO | Benzoyl peroxide |
| PTFE | Polytetrafluoroethylene |
| NiCr | Nickel-chromium |
| SiC | Silicon carbide |
| RH | Relative humidity |
| EPDM | Ethylene-propylene-diene |
| PP | Polypropylene |
References
- Muñoz-Pascual, S.; Saiz-Arroyo, C.; Vuluga, Z.; Corobea, M.C.; Rodriguez-Perez, M.A.J.P. Foams with Enhanced Ductility and Impact Behavior Based on Polypropylene. Composites 2020, 12, 943. [Google Scholar]
- Kiss, G.; Rusu, G.; Peter, F.; Tănase, I.; Bandur, G.J.P. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Solares, S.; Cimavilla-Roman, P.; Rodriguez-Perez, M.A.; Pinto, J.J.P. Non-Invasive Approaches for the Evaluation of the Functionalization of Melamine Foams with In-Situ Synthesized Silver Nanoparticles. Polymers 2020, 12, 996. [Google Scholar] [CrossRef]
- Pinto, J.; Barroso-Solares, S.; Magrì, D.; Palazon, F.; Lauciello, S.; Athanassiou, A.; Fragouli, D.J.P. Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles. Polymers 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.C.; Roslan, R.A.; Lau, W.J.; Gürsoy, M.; Karaman, M.; Jullok, N.; Ismail, A.F.J.P. A Green Approach to Modify Surface Properties of Polyurethane Foam for Enhanced Oil Absorption. Polymers 2020, 12, 1883. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodríguez-Pérez, M.A. Towards a new generation of polymeric foams: PMMA nanocellular foams with enhanced physical properties. Polymer 2015, 63, 116–126. [Google Scholar] [CrossRef]
- Pinto, J.; Escudero, J.; Solórzano, E.; Rodriguez-Perez, M.A. A novel route to produce structural polymer foams with a controlled solid skin-porous core structure based on gas diffusion mechanisms. Materials 2020, 22, 822–832. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Thanomsilp, C.; Suwantong, O. A review: Starch-based composite foams. Compos. Part A Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Demitri, C.; Raucci, M.G.; Giuri, A.; De Benedictis, V.M.; Giugliano, D.; Calcagnile, P.; Sannino, A.; Ambrosio, L. Cellulose-based porous scaffold for bone tissue engineering applications: Assessment of hMSC proliferation and differentiation. J. Biomed. Mater. Res. Part A 2016, 104, 726–733. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Gardziella, A.; Pilato, L.A.; Knop, A. Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Zhang, F.; Zhou, T.; Liu, Y.; Leng, J. Microwave synthesis and actuation of shape memory polycaprolactone foams with high speed. Sci. Rep. 2015, 5, 11152. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 Nanosheets Grown on Nitrogen-Doped Carbon Foams as an Advanced Electrode for Supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Kim, B.G. Development of microwave foaming method for phenolic insulation foams. J. Mater. Process. Technol. 2008, 201, 716–719. [Google Scholar] [CrossRef]
- Davies, G.; Zhen, S. Metallic foams: Their production, properties and applications. J. Mater. Sci. 1983, 18, 1899–1911. [Google Scholar] [CrossRef]
- You, B.; Jiang, J.; Fan, S. Three-dimensional hierarchically porous all-carbon foams for supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 15302–15308. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.J.M.; Kemmere, M.F.; Keurentjes, J.T.F. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications. Green Chem. 2008, 10, 731–738. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Banhart, J. Aluminum foams: On the road to real applications. Mrs Bull. 2003, 28, 290–295. [Google Scholar] [CrossRef]
- Raps, D.; Hossieny, N.; Park, C.B.; Altstädt, V. Past and present developments in polymer bead foams and bead foaming technology. Polymer 2015, 56, 5–19. [Google Scholar] [CrossRef]
- Martini-Vvedensky, J.E.; Suh, N.P.; Waldman, F.A. Microcellular Closed Cell Foams and Their Method of Manufacture. U.S. Patent 44,736,65A, 25 September 1984. [Google Scholar]
- Sauceau, M.; Fages, J.; Common, A.; Nikitine, C.; Rodier, E. New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Prog. Polym. Sci. 2011, 36, 749–766. [Google Scholar] [CrossRef] [Green Version]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Farhan, S.; Wang, R.; Jiang, H.; Li, K.; Wang, C. A novel combination of simple foaming and freeze-drying processes for making carbon foam containing multiwalled carbon nanotubes. Ceram. Int. 2016, 42, 8980–8989. [Google Scholar] [CrossRef]
- Dong, S.; Ji, X.; Yu, M.; Xie, Y.; Zhang, D.; He, X. Direct synthesis of interconnected porous carbon nanosheet/nickel foam composite for high-performance supercapacitors by microwave-assisted heating. J. Porous Mater. 2017, 25, 923–933. [Google Scholar] [CrossRef]
- Rezvanpanah, E.; Ghaffarian Anbaran, S.R.; Maio, E.D. Carbon nanotubes in microwave foaming of thermoplastics. Carbon 2017, 125, 32–38. [Google Scholar] [CrossRef]
- Ferrari-John, R.; Batchelor, A.; Katrib, J.; Dodds, C.; Kingman, S. Understanding selectivity in radio frequency and microwave sorting of porphyry copper ores. Int. J. Miner. Process. 2016, 155, 64–73. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.; Mavrofidis, S.; Kingman, S.; Miles, N. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, C.; Mei, B.; Yuan, R.; Fu, Z. Research on the technology and the mechanical properties of the microwave processing of polymer. J. Mater. Process. Technol. 2003, 137, 156–158. [Google Scholar] [CrossRef]
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Razza, F.; Degli Innocenti, F.; Dobon, A.; Aliaga, C.; Sanchez, C.; Hortal, M. Environmental profile of a bio-based and biodegradable foamed packaging prototype in comparison with the current benchmark. J. Clean. Prod. 2015, 102, 493–500. [Google Scholar] [CrossRef]
- Ahmad Zauzi, N.S.; Ariff, Z.M.; Khimi, S.R. Foamability of Natural Rubber via Microwave Assisted Foaming with Azodicarbonamide (ADC) as Blowing Agent. Mater. Today Proc. 2019, 17, 1001–1007. [Google Scholar] [CrossRef]
- Xu, H.; Xu, P.; Wang, D.; Yang, Y.; Wang, X.; Wang, T.; An, W.; Xu, S.; Wang, Y.-Z. A dimensional stable hydrogel-born foam with enhanced mechanical and thermal insulation and fire-retarding properties via fast microwave foaming. Chem. Eng. J. 2020, 399, 125781. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, L.; Peng, Y.; Yang, Q.; Liu, Y.; Wu, Q.; Tang, Y.; Zhu, H.; Dai, L.; Zeng, Z.; et al. Ex-situ catalytic fast pyrolysis of soapstock for aromatic oil over microwave-driven HZSM-5@SiC ceramic foam. Chem. Eng. J. 2020, 402, 126239. [Google Scholar] [CrossRef]
- Kang, Y.; Li, W.; Ma, T.; Huang, X.; Mo, Y.; Chu, Z.; Zhang, Z.; Feng, G. Microwave-constructed honeycomb architectures of h-BN/rGO nano-hybrids for efficient microwave conversion. Compos. Sci. Technol. 2019, 174, 184–193. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Chien, Y.-C. Field Study of in situ remediation of petroleum hydrocarbon contaminated soil on site using microwave energy. J. Hazard. Mater. 2012, 199, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Minay, E.; Veronesi, P.; Cannillo, V.; Leonelli, C.; Boccaccini, A. Control of pore size by metallic fibres in glass matrix composite foams produced by microwave heating. J. Eur. Ceram. Soc. 2004, 24, 3203–3208. [Google Scholar] [CrossRef]
- Jimenez, G.D.; Monti, T.; Titman, J.J.; Hernandez-Montoya, V.; Kingman, S.; Binner, E. New insights into microwave pyrolysis of biomass: Preparation of carbon-based products from pecan nutshells and their application in wastewater treatment. J. Anal. Appl. Pyrolysis 2017, 124, 113–121. [Google Scholar] [CrossRef]
- John, R.; Batchelor, A.; Ivanov, D.; Udoudo, O.; Jones, D.; Dodds, C.; Kingman, S. Understanding microwave induced sorting of porphyry copper ores. Miner. Eng. 2015, 84, 77–87. [Google Scholar] [CrossRef]
- Metaxas, A.A.; Meredith, R.J. Industrial Microwwave Heating; IET Peter Peregrinus Ltd.: London, UK, 1983. [Google Scholar]
- Calles-Arriaga, C.A.; López-Hernández, J.; Hernández-Ordoñez, M.; Echavarría-Solís, R.A.; Ovando-Medina, V.M. Thermal characterization of microwave assisted foaming of expandable polystyrene. Ing. Investig. Tecnol. 2016, 17, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Fang, X.; Yao, D. Processing of composite polystyrene foam with a honeycomb structure. Polym. Eng. Sci. 2015, 55, 1494–1503. [Google Scholar] [CrossRef]
- Wu, K.; Park, H.-S.; Willert-Porada, M. Pyrolysis of polyurethane by microwave hybrid heating for the processing of NiCr foams. J. Mater. Process. Technol. 2012, 212, 1481–1487. [Google Scholar] [CrossRef]
- Peng, X.; Song, J.; Nesbitt, A.; Day, R. Microwave foaming of starch-based materials (II) thermo-mechanical performance. J.Cell. Plast. 2013, 49, 147–160. [Google Scholar] [CrossRef]
- Choe, J.; Kim, M.; Kim, J. A microwave foaming method for fabricating glass fiber reinforced phenolic foam. Compos. Struct. 2016, 152, 239–246. [Google Scholar] [CrossRef]
- Zhou, J.; Song, J.; Parker, R. Structure and properties of starch-based foams prepared by microwave heating from extruded pellets. Carbohydr. Polym. 2006, 63, 466–475. [Google Scholar] [CrossRef]
- Chevali, V.; Kandare, E. 13 Rigid biofoam composites as eco-efficient construction materials. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 275–304. [Google Scholar] [CrossRef]
- Obradovic, J.; Voutilainen, M.; Virtanen, P.; Lassila, L.; Fardim, P.J.M. Cellulose fibre-reinforced biofoam for structural applications. Materials 2017, 10, 619. [Google Scholar] [CrossRef] [Green Version]
- López-Gil, A.; Silva-Bellucci, F.; Velasco, D.; Ardanuy, M.; Rodriguez-Perez, M. Cellular structure and mechanical properties of starch-based foamed blocks reinforced with natural fibers and produced by microwave heating. Ind. Crop. Prod. 2015, 66, 194–205. [Google Scholar] [CrossRef]
- Song, S.A.; Oh, H.J.; Kim, B.G.; Kim, S.S. Novel foaming methods to fabricate activated carbon reinforced microcellular phenolic foams. Compos. Sci. Technol. 2013, 76, 45–51. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Rajendran, B.; Avadhani, G.S.; Ramalingam, C.; Kumar, A. Microwave-irradiation-assisted hybrid chemical approach for titanium dioxide nanoparticle synthesis: Microbial and cytotoxicological evaluation. Environ. Sci. Pollut. Res. 2016, 23, 12287–12302. [Google Scholar] [CrossRef] [PubMed]
- Lamiel, C.; Kumar, D.R.; Shim, J.-J. Microwave-assisted binder-free synthesis of 3D Ni-Co-Mn oxide nanoflakes@ Ni foam electrode for supercapacitor applications. Chem. Eng. J. 2017, 316, 1091–1102. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, H.; Chen, Y.; Wang, W.; Chen, Y.; Zhong, Y. Microwave-assisted synthesis of functionalized graphene on Ni foam as electrodes for supercapacitor application. Electrochim. Acta 2013, 108, 421–428. [Google Scholar] [CrossRef]
- Ou, X.; Xu, S.; Warnett, J.M.; Holmes, S.M.; Zaheer, A.; Garforth, A.A.; Williams, M.A.; Jiao, Y.; Fan, X. Creating hierarchies promptly: Microwave-accelerated synthesis of ZSM-5 zeolites on macrocellular silicon carbide (SiC) foams. Chem. Eng. J. 2017, 312, 1–9. [Google Scholar] [CrossRef]
- Demitri, C.; Giuri, A.; Raucci, M.G.; Giugliano, D.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Preparation and characterization of cellulose-based foams via microwave curing. Interface Focus 2014, 4, 20130053. [Google Scholar] [CrossRef]
- Thomas-Hillman, I.; Laybourn, A.; Dodds, C.; Kingman, S.W. Realising the environmental benefits of metal–organic frameworks: Recent advances in microwave synthesis. J. Mater. Chem. A. 2018, 6, 11564–11581. [Google Scholar] [CrossRef] [Green Version]
- Canencia, F.; Darder, M.; Aranda, P.; Fernandes, F.M.; Gouveia, R.F.; Ruiz-Hitzky, E. Conducting macroporous carbon foams derived from microwave-generated caramel/silica gel intermediates. J. Mater. Sci. 2017, 52, 11269–11281. [Google Scholar] [CrossRef]
- Haq, E.U.; Padmanabhan, S.K.; Licciulli, A. Microwave synthesis of thermal insulating foams from coal derived bottom ash. Fuel Process. Technol. 2015, 130, 263–267. [Google Scholar] [CrossRef]
- Peyda, S.; Morshedian, J.; Karbalaei-Bagher, M.; Baharvand, H.; Khorasani, M.T. A novel technique in the foaming process of EPDM/PP via microwave radiation: The effect of blend compatibilization and additive encapsulation. RSC Adv. 2016, 6, 81400–81407. [Google Scholar] [CrossRef]
- van der Sman, R.G.M.; Bows, J.R. Critical factors in microwave expansion of starchy snacks. J. Food Eng. 2017, 211, 69–84. [Google Scholar] [CrossRef]
- Hardy, Z.; Jideani, V.A. Foam-mat drying technology: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2560–2572. [Google Scholar] [CrossRef]
- Peng, X.; Song, J.; Nesbitt, A.; Day, R. Microwave foaming of starch-based materials (I) dielectric performance. J. Cell. Plast. 2013, 49, 245–258. [Google Scholar] [CrossRef]
- Kraus, S.; Schuchmann, H.P.; Gaukel, V. Factors Influencing the Microwave-Induced Expansion of Starch-Based Extruded Pellets under Vacuum. J. Food Process. Eng. 2014, 37, 264–272. [Google Scholar] [CrossRef]
- Kraus, S.; Sólyom, K.; Schuchmann, H.P.; Gaukel, V. Drying Kinetics and Expansion of Non-predried Extruded Starch-Based Pellets during Microwave Vacuum Processing. J. Food Process. Eng. 2013, 36, 763–773. [Google Scholar] [CrossRef]
- Chanvrier, H.; Chaunier, L.; Della Valle, G.; Lourdin, D. Flow and foam properties of extruded maize flour and its biopolymer blends expanded by microwave. Food Res. Int. 2015, 76, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Song, S.A.; Chung, Y.S.; Kim, S.S. The mechanical and thermal characteristics of phenolic foams reinforced with carbon nanoparticles. Compos. Sci. Technol. 2014, 103, 85–93. [Google Scholar] [CrossRef]
- Prociak, A.; Owski, S.; Bąk, S. Thermoplastic polyurethane foamed under microwave irradiation. Polimery 2012, 57, 786–790. [Google Scholar] [CrossRef]
- Hong, Y.; Fang, X.; Yao, D. Microwave processing of syntactic foam from an expandable thermoset/thermoplastic mixture. Polym. Eng. Sci. 2015, 55, 1818–1828. [Google Scholar] [CrossRef]
- Demitri, C.; Giuri, A.; De Benedictis, V.M.; Raucci, M.G.; Giugliano, D.; Sannino, A.; Ambrosio, L. Microwave-induced porosity and bioactivation of chitosan-PEGDA scaffolds: Morphology, mechanical properties and osteogenic differentiation. J. Tissue Eng. Regen. Med. 2017, 11, 86–98. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kobashi, M.; Kanetake, N. Production of the Al3Ti foam by microwave heating. In ICAA13 Pittsburgh; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1043–1047. [Google Scholar]
- Ruiz-Trejo, E.; Azad, A.K.; Irvine, J.T. A 60-second microwave-assisted synthesis of nickel foam and its application to the impregnation of porous scaffolds. J. Electrochem. Soc. 2015, 162, F273–F279. [Google Scholar] [CrossRef] [Green Version]
- Paul, R.; Voevodin, A.A.; Zemlyanov, D.; Roy, A.K.; Fisher, T.S. Microwave-Assisted Surface Synthesis of a Boron-Carbon-Nitrogen Foam and its Desorption Enthalpy. Adv. Funct. Mater. 2012, 22, 3682–3690. [Google Scholar] [CrossRef]
- Kolbitsch, C.; Link, M.; Petutschnigg, A.; Wieland, S.; Tondi, G. Microwave produced tannin-furanic foams. J. Mater. Sci. Res. 2012, 1, 84. [Google Scholar] [CrossRef] [Green Version]
- Moraru, C.; Kokini, J. Nucleation and expansion during extrusion and microwave heating of cereal foods. Compr. Rev. Food Sci. Food Saf. 2003, 2, 147–165. [Google Scholar] [CrossRef]
- CellMat. Microwave Foaming of Starch. Bioplastic Cellular Materials. Available online: http://cellmat.es/bioplastic-cellular-materials/ (accessed on 1 October 2020).
- Lee, G.H.; Park, B.K.; Lee, W.I. Microstructure and property characterization of flexible syntactic foam for insulation material via mold casting. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 169–176. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, Y.; Dong, A.; Li, Y. Preparation and Properties of C/C Hollow Spheres and the Energy Absorption Capacity of the Corresponding Aluminum Syntactic Foams. Materials 2018, 11, 997. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhao, Y.; Dong, A.; Li, Y. Mechanical properties of EPS filled syntactic foams prepared by VARTM. Compos. Part B Eng. 2018, 136, 126–134. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, H.-Y.; Lee, J.-R. Curing and thermal properties of a microwave radiation-curable epoxy/latent catalyst system. J. Ind. Eng. Chem. 2005, 11, 726–731. [Google Scholar]
- Jee, C.; Guo, Z.; Evans, J.; Özgüven, N. Preparation of high porosity metal foams. Metall. Mater. Trans. B 2000, 31, 1345–1352. [Google Scholar] [CrossRef]
- Peng, H.; Fan, Z.; Evans, J.; Busfield, J. Microstructure of ceramic foams. J. Eur. Ceram. Soc. 2000, 20, 807–813. [Google Scholar] [CrossRef]
- Chan, T.V.C.T.; Reader, H.C. Understanding Microwave Heating Cavities; Artech House Publishers: Norwood, MA, USA, 2000. [Google Scholar]
- Arimi, J.; Duggan, E.; O’Riordan, E.; O’Sullivan, M.; Lyng, J. Microwave expansion of imitation cheese containing resistant starch. J. Food Eng. 2008, 88, 254–262. [Google Scholar] [CrossRef]
- Sjöqvist, M.; Gatenholm, P. Effect of water content in potato amylopectin starch on microwave foaming process. J. Polym. Environ. 2007, 15, 43. [Google Scholar] [CrossRef]
- Boischot, C.; Moraru, C.; Kokini, J. Factors that influence the microwave expansion of glassy amylopectin extrudates. Cereal Chem. 2003, 80, 56. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Song, S.A.; Lee, Y.; Kim, Y.S.; Kim, S.S. Mechanical and thermal properties of carbon foam derived from phenolic foam reinforced with composite particles. Compos. Struct. 2017, 173, 1–8. [Google Scholar] [CrossRef]
- Prosperetti, A. A generalization of the Rayleigh–Plesset equation of bubble dynamics. Phys. Fluids 1982, 25, 409–410. [Google Scholar] [CrossRef]
- Tong, C.; Lund, D. Microwave heating of baked dough products with simultaneous heat and moisture transfer. J. Food Eng. 1993, 19, 319–339. [Google Scholar] [CrossRef]
- Khraisheh, M.; Cooper, T.; Magee, T. Microwave and air drying I. Fundamental considerations and assumptions for the simplified thermal calculations of volumetric power absorption. J. Food Eng. 1997, 33, 207–219. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Sait, H.H.; Salema, A.A. Microwave dielectric characterization of Saudi Arabian date palm biomass during pyrolysis and at industrial frequencies. Fuel 2015, 161, 239–247. [Google Scholar] [CrossRef]
- Lin, H.; Luo, H.; Huang, W.; Zhang, X.; Yao, G. Diffusion bonding in fabrication of aluminum foam sandwich panels. J. Mater. Process. Technol. 2016, 230, 35–41. [Google Scholar] [CrossRef]
- Chang, K.; Gao, J.-T.; Wang, Z.; Guo, Z.-C. Manufacturing 3-D open-cell aluminum foam via infiltration casting in a super-gravity field. J. Mater. Process. Technol. 2018, 252, 705–710. [Google Scholar] [CrossRef]
- Lara-Rodriguez, G.A.; Figueroa, I.A.; Suarez, M.A.; Novelo-Peralta, O.; Alfonso, I.; Goodall, R. A replication-casting device for manufacturing open-cell Mg foams. J. Mater. Process. Technol. 2017, 243, 16–22. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, N.; Wei, Q.; Zhang, L.; Li, H.; Luo, J.; Lin, C.-T.; Ma, L.; Zhou, K.; Yu, Z. Carbon nanotube-Cu foam hybrid reinforcements in composite phase change materials with enhanced thermal conductivity. Mater. Des. 2019, 172, 107709. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Sun, D.; Lin, T.; Huang, F. Nanoporous Carbon Foam for Water and Air Purification. ACS Appl. Nano Mater. 2020, 3, 1564–1570. [Google Scholar] [CrossRef]
- Menéndez, J.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zubair, M.; Shehzad, F.; Al-Harthi, M.A. Impact of modified graphene and microwave irradiation on thermal stability and degradation mechanism of poly (styrene-co-methyl meth acrylate). Thermochim. Acta 2016, 633, 48–55. [Google Scholar] [CrossRef]













| Method | Steps | Application | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Extrusion |
|
|
|
| [9,18] |
| Extrusion with supercritical fluids |
|
|
|
| [19,32] |
| Freeze-drying/solvent exchange |
|
|
|
| [19] |
| Microwave foaming |
|
|
|
| [33,34] |
| Batch foaming |
|
|
|
| [18,19] |
| Injection molding foaming |
|
|
|
| [18,19] |
| Precursor | Blowing Agent | Additives | Operating Condition | Foaming Steps | Foam Product Properties | Remarks/Microwave Benefits | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Power (Watt) | Time (s) | Product | Density (g/cm3) | Porosity | Compressive Strength (MPa)/Thermal Conductivity (W/m K) | ||||||
| Temple flour, superfine flour, purified wheat starch | Hydrocerol | Calcium chloride and sodium chloride, talc powder | - | 50–65 | Extrusion (12%–13% MC) Additive addition Microwave foaming | Temple Superfine Starch Temple/NaCl Temple/CaCl2 Temple/BIH Temple/talc (2.2%) | 0.15 0.114 0.139 0.095 0.092 0.144 0.188 | 0.314/- 0.180/- 0.161/- 0.250/- 0.174/- 0.334/- 0.489/- | Effective microwave absorption due to the presence of salts led to the increase in heating rate, low moisture loss due to diffusion, and larger cell sizes. The mechanical properties of microwave foamed foam were found close to that of the EPS block. | [48] | |
| Purified wheat starch wheat flour | Water | Glycerol, polyvinyl alcohol | Extrusion and addition of additives Microwave foaming | - | - | - | Glycerol enhanced microwave absorption efficiency and high heating rate at lower microwave power Presence of additives constraint to foam cell growth during microwave foaming | [64] | |||
| Purified wheat starch wheat flour | Water | Glycerol, polyvinyl alcohol | 200 | - | - | - | Reduction in expansion during microwave foaming of starch materials containing glycerol | [46] | |||
| Native corn starch | Water | None | 400 600 800 | 120 120 120 | Extrusion (MC 41%) Vacuum foaming and drying | 2020 pores 1018 pores 1970 pores | Higher the microwave power leads to a higher number of vapor bubbles nucleated and showed increase in the volume expansion microwave Vacuum expansion allows an indirect expansion with lower time and energy consumption | [65] | |||
| Native corn starch | Water | None | 400 600 800 | 120 120 120 | Extrusion (MC 0.695 kg/kg) Vacuum foaming and drying | 0.82 EI 0.97 EI 1.17 EI | The volume of starch-based pellets significantly increased with increasing microwave power; effective moisture diffusivities increased with an increasing microwave power. | [66] | |||
| Native wheat starch | Water | Barley straw fibers, cardoon waste, and grape waste. Barley straw fibers | 900 | 50 | Extrusion with and without additives Microwave foaming of sheets in PTFE mold | Starch Starch/Barley (95:5) Starch/Grape (95:5) Starch Cardoon (95:5) | 0.292 0.347 0.301 0.303 | cell sizes > 0.5 | 0.87/- 2.18/- 2.03/- 1.92/- | The microwave foaming process allowed the continuous production of foam blocks (without joining between pellets). This leads to about an 800-time increase in mechanical performance (stiffness and strength) compared to pellets microwave foaming. | [51] |
| Maize flour | Zein biopolymers | 1000 | 15 | Extrusion with and without additives (MC 26%) Microwave foaming | Starch Starch/zein/55/5 Starch/zein/85/15 Starch/zein/70/30 | 0.16 0.2 0.22 0.44 | <100 um < 100 um < 100 um > 200 um | Foams from microwaved polymer mixture exhibited finer cellular structure compared to directly expanded material. | [67] | ||
| Carboxymethylcellulose | Pluronic | Polyethylene glycol diacrylate | 900 | 105 | Mixing of polymers and blowing agent Microwave foaming Drying | The microwave method effectively induced thermo-polymerization with time and energy savings. The foam is a hierarchical structure having open porosity of different sizes. | [57] | ||||
| Resole | Air bubbles | - | 12,000 | 3–20 | Mixing of resoling, hardener, and air bubbles using an impeller Microwave foaming | Phenolic foam | 0.12 | 100–150 um dia | -/0.029 | Microwave significantly decreased the content of H2O (the byproduct of cure reaction), which leads to low conductivity foams compared to conventional phenolic foams. | [15] |
| Resole-type phenolic resins | Air bubbles | AC powder | 12,000 | Mixing the resole and accelerators with or without the AC using an impeller chemical cure reaction Microwave foaming | Phenolic foam Phenolic/AC (1 wt%) | 0.127 0.103 | 233.8 um 169.6 um | 1.68/0.064 2.17/−0.071 | Microwave radiation helps chemical interaction between the phenolic resin and AC during foaming that induces a robust interface and thus resulted in a firmer foam. | [52] | |
| Resole-type phenolic resins | Air bubbles | MWCNT and graphene | 12,000 | 20 | Mixing the resole and accelerators with or without the additives using an impeller chemical cure reaction Microwave foaming | Phenolic foam 0.5 wt% MWCNT 1.0 wt% MWCNT 0.5 wt% Graphen 1.0 wt% Graphene e | 0.065 0.050 0.065 0.072 0.048 | 94.4% 95.3% 93.8% 93.5% 95.4% | 0.11/- 0.14/- 0.13/- 0.14/- 0.17/- | The cure starting point of the particle-reinforced phenolic resin occurred sooner than that of the neat phenolic resin because nanoparticles catalyze the cure reaction of the phenolic resin at lower temperatures due to microwaves. | [68] |
| Resole-type phenolic resin | Air bubbles | Chopped glass fiber, ethanol, PTSA catalyst | 1000 | 60 | Resin preparation and air bubbles entrapping Acid catalyst mixing Foaming and curing | Phenolic foam with 12% ethanol and 3 or 6 wt% catalyst | 0.035 | 0.148/0.039 | Highly uniform phenolic foam was fabricated with 12 wt% ethanol using closed mold microwave foaming process. | [47] | |
| BA | SS | 900 | 240 | Missing of BA and SS Microwave foaming in Teflon mold Removal of moisture content | BA:SS(4:6) BA:SS(5:5) BA:SS(6:4) BA:SS(7:3) | 0.61 0.64 1.1 1.3 | 72.64% 71.3% 50.67% 41.7% | 3.55/0.075 3/0.07 6.23/0.09 3.67/0.091 | Microwave heating initiates the cross-linking of silicates groups, which form an impermeable skin and leads to a highly porous scaffold during foaming. | [60] | |
| TPUR | ADC | CB | 500 | 180 (4 cycles) | Extrusion with blowing agent and CB Microwave foaming | TPUR + ADC TPUR + ADC + CB | 0.564 0.050 | - - - | The presence of CB in TPUR showed effective microwave heating due to an increase in microwave absorbance, which gave more fine particles. Carbon black additive improves the cell structure and, increases the apparent density but significantly worsens its mechanical properties. | [69] | |
| EPS | Ethanol, hydrogen peroxide, ethanol/water | - | 950 | 180 | Injection of solvents in EPS beads Microwave foaming | - | - | - | -/0.029 | The better temperature distribution was achieved with hydrogen peroxides using microwave heating. | [43] |
| Epoxy resin and EPS | Hardener | 950 | - | Mixing of epoxy resin hardener and EPS beads Microwave foaming Curing | EPS–Epoxy 5% (w/w) EPS–Epoxy 45% (w/w) | 0.84 0.28 | - | The microwave foaming process successfully molded the syntactic foam with sophisticated geometry and smooth surfaces. | [70] | ||
| Unexpanded EPS microspheres | Pentane | Phenolic resin | 1000 | Mixing of EPS, pentane, and phenolic resin Microwave foaming Post curing | Neat EPS foam Composite EPS foam | 0.043 0.093 | 3.35/- 5.80/- | Effective expansion of EPS-syntactic foam at high EPS loading via microwave heating Improved fire-resistant properties due to the formation of a honeycomb structure of composite foam compared to neat polystyrene foam. | [44] | ||
| EPDM, PP | ADC | Urea, paraformaldehyde, iron oxide | 900 | 720 | The blending of all materials preparation of microcapsules microwave irradiation | EPDM/PP | 0.61 | - | The microwave technique allows the production of EPDM/PP foam with uniform voids and greater cell sizes of 435 microns, which is almost double produced using the conventional technique. | [61] | |
| CS, PEGDA | Pluronic | - | 800–1000 | 45–240 | Mixing of materials and foaming agent Microwave heating | 30P70CS1.5 40P60CS1.5 | 78.90 60.76 | Microwave heating allows a homogenous heating process and effectively produced a highly porous interconnected scaffold. | [71] | ||
| Titanium and aluminum | Boron carbide (B4C) | 200–450 | A blending of aluminum, titanium and B4C uniaxial pressing to make a cylindrical precursor microwave heating | Al3Ti Al3Ti + 10% B4C Al3Ti + 10% B4C | - | 40% 60% 61% | Microwave heating succeeded in ignition combustion synthesis reaction to produce Al3Ti foam. | [72] | |||
| Nickel nitrate | - | Glycine | 1000 | 60 | Mixing glycine with a nitrate solution Microwave foaming | Nickel foam | - | 40% | Microwave showed high potential to provide a homogenous and increased impregnation rate in the porous scaffold with no surface structure damage. The nanoparticle–microwave interaction caused interconnected nanoparticles, resulting in a percolating network inside the scaffold. | [73] | |
| Graphitic carbon foam (70% porosity) | Boric acid and urea | 400 | 300–18,00 | Microwave heating of carbon foam in the presence of additives Vacuum drying Annealing at 500–1100 °C | Boron carbon nitride foam | Microwave treatment effectively activated surface chemical reactions between carbon foam, boric acid, and urea. | [74] | ||||
| Sucrose | Water | Silica gel | 850 | 180 | Mixing of sucrose, silica gel, and water Microwave irradiation thermal treatment at 800 °C under nitrogen atmosphere | Carbon foam | 0.17 | 97% (5–6 nm) | Highly porous carbon/silica foam produced using microwave without any blowing agent. | [59] | |
| PCL | DCM | BPO | 700 | 240 | Dissolve PCL in DCM with BPO and mix to homogenous Microwave foaming | PCL-10 wt% BPOPCL-15 wt%BPO | 0.40 0.37 | 63.55% 66.39% | The microwave heating considerably increased the actuation efficiency compared to other heating methods. | [13] | |
| Mimosa tannin extract | Water, furfuryl alcohol, methanol diethylether | - | 600 | 120 | Mixing of all solvent and tannin extract Microwave foaming | tannin-furanic foams | 0.11 | 0.44/- | The microwave foaming method of tannic-furanic foam allows a substantially reduced hardening rate of the polymer and the solvent’s blowing. This facilitates faster polymerization and water evaporation and decreased the blowing agent consumption. | [75] | |
| Opportunity | Challenge | |
|---|---|---|
| Environmental | Microwaves can selectively heat water (high dielectric loss) within the matrix, allowing use as a sustainable blowing agent. | Stable addition of water into a polymer matrix |
| Product quality | Produce controlled pore structures enabled by instant control of heating Higher expansion volume; more product per unit of precursor | Electromagnetic design of homogeneous electric field distribution at large scale |
| Distributed manufacture based on different (waste-derived) feedstocks/precursors, e.g., packaging | Microwave technologies scalable/can fit in a container enabling small scale local/mobile processing | Understanding sensitive of systems to feedstock variation and incorporating into the electromagnetic design of a system |
| Reduced processing time and energy consumption | Microwaves apply rapid selective and volumetric heating. | Understanding whether reduced OPEX can offset increased CAPEX for microwave systems |
| New composites, e.g., polymer syntactic foams | Expansion of higher dielectric loss beads within a microwave transparent matrix | Controlled expansion and hardening/curing of materials |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubair, M.; Ferrari, R.; Alagha, O.; Mu’azu, N.D.; Blaisi, N.I.; Ateeq, I.S.; Manzar, M.S. Microwave Foaming of Materials: An Emerging Field. Polymers 2020, 12, 2477. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112477
Zubair M, Ferrari R, Alagha O, Mu’azu ND, Blaisi NI, Ateeq IS, Manzar MS. Microwave Foaming of Materials: An Emerging Field. Polymers. 2020; 12(11):2477. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112477
Chicago/Turabian StyleZubair, Mukarram, Rebecca Ferrari, Omar Alagha, Nuhu Dalhat Mu’azu, Nawaf I. Blaisi, Ijlal Shahrukh Ateeq, and Mohammad Saood Manzar. 2020. "Microwave Foaming of Materials: An Emerging Field" Polymers 12, no. 11: 2477. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112477