Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(ester-Carbonate)s Bearing Pendant Primary Amine in the Main Chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Experimental Methodology
2.3.1. Synthesis of Cyclic Carbonates Bearing Potential Primary Amino Groups
2.3.2. Typical Synthesis of Random Copolymer Poly(ε-caprolactone-ran-tert-butyl (2-oxo-1,3-dioxan-5-yl)carbamate) (P(CL-ran-TBODC))
2.3.3. Preparation of Poly(ester-carbonate)s Bearing Primary Amine
2.3.4. Antimicrobial Activity
2.3.5. Hemolysis
2.3.6. Cyto-Compatibility
3. Results and Discussion
3.1. Synthesis of Cyclic Carbonate TBODC
3.2. Random Copolymerization of CL and TBODC
3.3. Deprotection of P(CL-ran-TBODC)
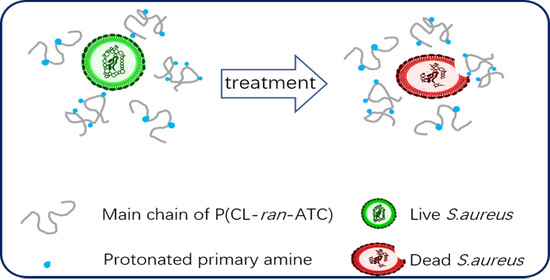
3.4. Antimicrobial Property, Hemolytic Activity, and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: The importance of interfacial activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. [Google Scholar] [CrossRef] [Green Version]
- Barman, S.; Konai, M.M.; Samaddar, S.; Haldar, J. Amino Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-Resistant Acinetobacter baumannii with No Detectable Resistance. ACS Appl. Mater. Interfaces 2019, 11, 33559–33572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial cationic polymers: From structural design to functional control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, D.; Shaw, P.D. (Eds.) Antibiotics Volume I Mechanism of Action; Springer: Berlin/Heidelberg, Germany, 1967; ISBN 978-3-662-37649-2. [Google Scholar]
- Epand, R.F.; Mowery, B.P.; Lee, S.E.; Stahl, S.S.; Lehrer, R.I.; Gellman, S.H.; Epand, R.M. Dual Mechanism of Bacterial Lethality for a Cationic Sequence-Random Copolymer that Mimics Host-Defense Antimicrobial Peptides. J. Mol. Biol. 2008, 379, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Ng, V.W.L.; Ke, X.; Lee, A.L.Z.; Hedrick, J.L.; Yang, Y.Y. Synergistic co-delivery of membrane-disrupting polymers with commercial antibiotics against highly opportunistic bacteria. Adv. Mater. 2013, 25, 6730–6736. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-pérez, A.; Calo-mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65, 46–62. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.L.Z.; Ng, V.W.L.; Wang, W.; Hedrick, J.L.; Yang, Y.Y. Block copolymer mixtures as antimicrobial hydrogels for biofilm eradication. Biomaterials 2013, 34, 10278–10286. [Google Scholar] [CrossRef]
- Kuroki, A.; Sangwan, P.; Qu, Y.; Peltier, R.; Sanchez-Cano, C.; Moat, J.; Dowson, C.G.; Williams, E.G.L.; Locock, K.E.S.; Hartlieb, M.; et al. Sequence Control as a Powerful Tool for Improving the Selectivity of Antimicrobial Polymers. ACS Appl. Mater. Interfaces 2017, 9, 40117–40126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinazo, A.; Petrizelli, V.; Bustelo, M.; Pons, R.; Vinardell, M.P.; Mitjans, M.; Manresa, A.; Perez, L. New cationic vesicles prepared with double chain surfactants from arginine: Role of the hydrophobic group on the antimicrobial activity and cytotoxicity. Colloids Surfaces B Biointerfaces 2016, 141, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Wang, H.; Ji, T.; Anderson, G.; Nie, G.; Zhao, Y. Biodegradable cationic epsilon-poly-L-lysine-conjugated polymeric nanoparticles as a new effective antibacterial agent. Sci. Bull. 2015, 60, 216–226. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, C.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial silica particles synthesized via ring-opening grafting of cationic amphiphilic cyclic carbonates: Effects of hydrophobicity and structure. Polym. Chem. 2016, 7, 2192–2201. [Google Scholar] [CrossRef]
- Yuin, Z.; Cheng, J.; Huang, Y.; Xu, K.; Ji, Z.; Fan, W. Biomaterials Effect of stereochemistry, chain length and sequence pattern on antimicrobial properties of short synthetic β -sheet forming peptide amphiphiles. Biomaterials 2014, 35, 1315–1325. [Google Scholar] [CrossRef]
- Punia, A.; He, E.; Lee, K.; Banerjee, P.; Yang, N.L. Cationic amphiphilic non-hemolytic polyacrylates with superior antibacterial activity. Chem. Commun. 2014, 50, 7071–7074. [Google Scholar] [CrossRef]
- Engler, A.C.; Tan, J.P.K.; Ong, Z.Y.; Coady, D.J.; Ng, V.W.L.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial polycarbonates: Investigating the impact of balancing charge and hydrophobicity using a same-centered polymer approach. Biomacromolecules 2013, 14, 4331–4339. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Hisey, B.; Harrison, T.D.; Trant, J.F.; Gillies, E.R.; Ragogna, P.J. Surprising Antibacterial Activity and Selectivity of Hydrophilic Polyphosphoniums Featuring Sugar and Hydroxy Substituents. Angew. Chem. Int. Ed. 2018, 57, 12707–12710. [Google Scholar] [CrossRef]
- Palermo, E.F.; Lienkamp, K.; Gillies, E.R.; Ragogna, P.J. Antibacterial Activity of Polymers: Discussions on the Nature of Amphiphilic Balance. Angew. Chem. Int. Ed. 2019, 58, 3690–3693. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Pascual, A.; Tan, J.P.K.; Yuen, A.; Chan, J.M.W.; Coady, D.J.; Mecerreyes, D.; Hedrick, J.L.; Yang, Y.Y.; Sardon, H. Broad-Spectrum Antimicrobial Polycarbonate Hydrogels with Fast Degradability. Biomacromolecules 2015, 16, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Exley, S.E.; Paslay, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Ergene, C.; Palermo, E.F. Cationic Poly(benzyl ether)s as Self-Immolative Antimicrobial Polymers. Biomacromolecules 2017, 18, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Paslay, L.C.; Abel, B.A.; Brown, T.D.; Koul, V.; Choudhary, V.; McCormick, C.L.; Morgan, S.E. Antimicrobial Poly(methacrylamide) Derivatives Prepared via Aqueous RAFT Polymerization Exhibit Biocidal Efficiency Dependent upon Cation Structure. Biomacromolecules 2012, 13, 2472–2482. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Ng, V.W.L.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable hydrogels from triblock copolymers of vitamin E-functionalized polycarbonate and poly(ethylene glycol) for subcutaneous delivery of antibodies for cancer therapy. Adv. Funct. Mater. 2014, 24, 1538–1550. [Google Scholar] [CrossRef]
- Chin, W.; Yang, C.; Wee, V.; Ng, L.; Huang, Y.; Cheng, J.; Tong, Y.W.; Coady, D.J.; Fan, W.; Hedrick, J.L.; et al. Biodegradable Broad-Spectrum Antimicrobial Polycarbonates: Investigating the Role of Chemical Structure on Activity and Selectivity. Macromolecules 2013, 46, 8797–8807. [Google Scholar] [CrossRef]
- Tan, J.P.K.; Gao, S.; Hedrick, J.L.; Zhang, Y.; Guo, X.D.; Li, L.; Yang, Y.; Fukushima, K.; Wang, H.; Yang, C.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar]
- Yang, C.; Krishnamurthy, S.; Liu, J.; Liu, S.; Lu, X.; Coady, D.J.; Cheng, W.; De Libero, G.; Singhal, A.; Hedrick, J.L.; et al. Broad-Spectrum Antimicrobial Star Polycarbonates Functionalized with Mannose for Targeting Bacteria Residing inside Immune Cells. Adv. Healthc. Mater. 2016, 5, 1272–1281. [Google Scholar] [CrossRef]
- Tan, J.P.K.; Coady, D.J.; Sardon, H.; Yuen, A.; Gao, S.; Lim, S.W.; Liang, Z.C.; Tan, E.W.; Venkataraman, S.; Engler, A.C.; et al. Broad Spectrum Macromolecular Antimicrobials with Biofilm Disruption Capability and In Vivo Efficacy. Adv. Healthc. Mater. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Qiang, N.; Yang, W.; Li, L.; Dong, P.; Zhu, J.; Wang, T.; Zeng, C.; Quan, D. Synthesis of pendent carboxyl-containing poly(ε-caprolactone-co- β-malic acid)-block-poly(l-lactide) copolymers for fabrication of nano-fibrous scaffolds. Polymer 2012, 53, 4993–5001. [Google Scholar] [CrossRef]
- Alarçin, E.; Lee, T.Y.; Karuthedom, S.; Mohammadi, M.; Brennan, M.A.; Lee, D.H.; Marrella, A.; Zhang, J.; Syla, D.; Zhang, Y.S.; et al. Injectable shear-thinning hydrogels for delivering osteogenic and angiogenic cells and growth factors. Biomater. Sci. 2018, 6, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, K.; Reghu, S.; Lin, Y.; Chan-Park, M.B.; Liu, X.W. Synthesis of Antibacterial Glycosylated Polycaprolactones Bearing Imidazoliums with Reduced Hemolytic Activity. Biomacromolecules 2019, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Tsumura, T.; Horibe, M.; Nakao, M. Catalytic asymmetric ring-opening of σ-symmetric cyclic carbonates with chiral bronsted acid catalysts. Synlett 2013, 24, 2302–2304. [Google Scholar] [CrossRef]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briançon, S.; Lantéri, P. Determination of poly(ε-caprolactone) solubility parameters: Application to solvent substitution in a microencapsulation process. Int. J. Pharm. 2010, 383, 236–243. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, Q.; Lyu, R.; Yu, L.; Su, S.; Du, F.S.; Li, Z.C. Synthesis of a ROS-responsive analogue of poly(ε-caprolactone) by the living ring-opening polymerization of 1,4-oxathiepan-7-one. Polym. Chem. 2018, 9, 4574–4584. [Google Scholar] [CrossRef]
- Li, L.; Zhai, H.; Wang, T.; Qiu, X.; Qiang, N.; Dong, P.; Bai, Y.; Peng, A.Y.; Quan, D. Bromine-functionalized poly(carbonate-co-lactide)s: Synthesis, characterization and post-polymerization functionalization. Polymer 2019, 180, 121705. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Coady, D.J.; Tan, J.P.K.; Li, Y.; Chan, J.M.W.; Yang, Y.Y.; Hedrick, J.L. Design and synthesis of biodegradable grafted cationic polycarbonates as broad spectrum antimicrobial agents. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Ng, V.W.L.; Tan, J.P.K.; Leong, J.; Voo, Z.X.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial polycarbonates: Investigating the impact of nitrogen-containing heterocycles as quaternizing agents. Macromolecules 2014, 47, 1285–1291. [Google Scholar] [CrossRef]







| Entry | fTBODC/ε-CLb | 1H NMR | GPC e | DSC | |||
|---|---|---|---|---|---|---|---|
| Fc | Mn/kDa d | Mn/kDa | Đ | Tg/°C | Tm/°C | ||
| 1 | 10/90 | 7.1 | 8.3 | 8.7 | 1.26 | −47.15 | 49.54 |
| 2 | 25/75 | 22.8 | 10.7 | 14.9 | 1.58 | −40.73 | 36.53 |
| 3 | 50/50 | 50.9 | 3.9 | 17.7 | 1.30 | −34.59 | N/A |
| 4 | 100/0 | N/A | 4.8 | 3.3 | 1.10 | 48.45 | N/A |
| 5 | 0/100 | N/A | 12.2 | 8.3 | 1.40 | /f | 63.50 |
| Polymer | HLR a | MIC (μg mL−1) | Hemolytic Activity (H4000) b | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| S. aureus (Gram +) | E. coli (Gram −) | ||||
| P(CL-ran-ATC 7.1%) | 7.1 | NA c | NA c | 2.16% | /d |
| P(CL-ran-ATC 22.8%) | 22.8 | 3500 | 4000 | 1.53% | 25.8 ± 1.8 |
| P(CL-ran-ATC 50.9%) | 50.9 | 2000 | 3000 | 1.41% | 38.3 ± 1.2 |
| PATC | / | 4000 | NA c | 0.74% | 7.7 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Feng, J.; Li, S.; Sun, T.; Shi, Q.; Xie, X. Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(ester-Carbonate)s Bearing Pendant Primary Amine in the Main Chain. Polymers 2020, 12, 2640. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112640
Dong P, Feng J, Li S, Sun T, Shi Q, Xie X. Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(ester-Carbonate)s Bearing Pendant Primary Amine in the Main Chain. Polymers. 2020; 12(11):2640. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112640
Chicago/Turabian StyleDong, Peng, Jing Feng, Sujuan Li, Tingli Sun, Qingshan Shi, and Xiaobao Xie. 2020. "Synthesis, Characterization, and Antimicrobial Evaluation of Random Poly(ester-Carbonate)s Bearing Pendant Primary Amine in the Main Chain" Polymers 12, no. 11: 2640. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112640