Facile Synthesis of Ultrahigh Molecular Weight Poly(Methyl Methacrylate) by Organic Halides in the Presence of Palladium Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Pd NPs
2.3. Synthesis of Ultrahigh Molecular Weight PMMA Using Organic Halides as Initiators in the Presence of Pd NPs
2.4. Synthesis of PMMA by Free Radical Polymerization
2.5. Synthesis of PMMA by Atom Transfer Radical Polymerization
2.6. Characterization
3. Result and Discussion
3.1. Characterization of Pd NPs
3.2. Synthesis of Ultrahigh Molecular Weight PMMA Using EBiB as an Initiator in the Presence of Pd NPs
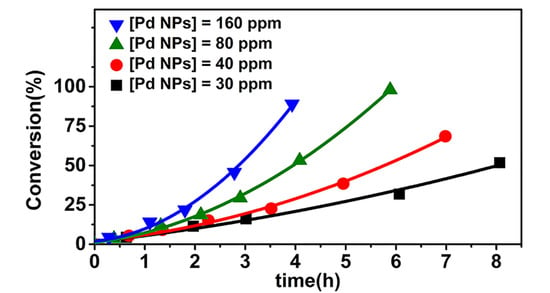
3.3. Kinetics of the Polymerization of MMA Initiated by EBiB in the Presence of Pd NPs
3.4. End Group Analysis of PMMA
3.5. Mechanistic Investigation of the Polymerization Initiated by EBiB in the Presence of Pd NPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Henri, L. Thermohygroelastic Properties of Polymethylmethacrylate; Technical Note PR-TN 2007/00440; Koninklijke Philips Electronics N.V.: Eindhoven, The Netherlands, 2007; pp. 11–13. [Google Scholar]
- Martin, J.R.; Johnson, J.F.; Cooper, A.R. Mechanical properties of polymers: The influence of molecular weight and molecular weight distribution. J. Macromol. Sci. Rev. Macromol. Chem. 1972, C8, 57–199. [Google Scholar] [CrossRef]
- Laius, L.A.; Kuvshinskii, E.V. Effect of molecular weight on strength and deformation characteristics of oriented amorphous polymers. Mekh. Polim. 1967, 3, 579–585. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Patra, B.N. [Cp2TiCl2] catalyzed polymerization in water: Polymerization of methylmethacrylate to a high molecular weight polymer. Polymer 2004, 45, 3111–3114. [Google Scholar] [CrossRef]
- Patra, B.N.; Bhattacharjee, M. Early transition metal catalyzed aqueous emulsion copolymerization: Copolymerization of styrene and methyl methacrylate by Cp2TiCl2 in aqueous medium. J. Polym. Sci. Pol. Chem. 2005, 43, 3707–3710. [Google Scholar] [CrossRef]
- Patra, B.N.; Bhattacharjee, M. Synthesis of high molecular weight polystyrene and poly(methyl methacrylate) with low polydispersity by [Cp2ZrCl2] catalyzed aqueous polymerization. J. Polym. Sci. Pol. Chem. 2005, 43, 3797–3803. [Google Scholar] [CrossRef]
- Granel, C.; Dubois, P.; Jerome, R.; Teyssie, P. Controlled radical polymerization of methacrylic monomers in the presence of a bis(ortho-chelated) arylnickel(II) complex and different activated alkyl halides. Macromolecules 1996, 29, 8576–8582. [Google Scholar] [CrossRef]
- Nishikawa, T.; Kamigaito, M.; Sawamoto, M. Living radical polymerization in water and alcohols: Suspension polymerization of methyl methacrylate with RuCl2(PPh3)3 complex1. Macromolecules 1999, 32, 2204–2209. [Google Scholar] [CrossRef]
- Osada, Y.; Bell, A.T.; Shen, M. Plasma-initiated polymerization of methyl methacrylate. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 309–311. [Google Scholar] [CrossRef]
- Johnson, D.R.; Osada, Y.; Bell, A.T.; Shen, M. Studies of the mechanism and kinetics of plasma-initiated polymerization of methyl methacrylate. Macromolecules 1981, 14, 118–124. [Google Scholar] [CrossRef]
- Tsai, Y.; Marrero, T.R.; Yasuda, H.K. Plasma polymerization for self-curing PMMA bone cement. J. Appl. Polym. Sci. 1994, 54, 1773–1779. [Google Scholar] [CrossRef]
- Yasuda, H.; Bumgarner, M.O.; Marsh, H.C.; Morosoff, N. Plasma polymerization of some organic compounds and properties of the polymers. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 195–224. [Google Scholar] [CrossRef]
- Yasuda, H.; Hsu, T. Some aspects of plasma polymerization investigated by Pulsed R.F. Discharge. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 81–97. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Control1ed “living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, M.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef]
- Grimaud, T.; Matyjaszewski, K. Controlled/“living” radical polymerization of methyl methacrylate by atom transfer radical polymerization. Macromolecules 1997, 30, 2216–2218. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Wei, M.; Xia, J.; McDermott, N.E. Controlled/“living” radical polymerization of styrene and methyl methacrylate catalyzed by Iron complexes1. Macromolecules 1997, 30, 8161–8164. [Google Scholar] [CrossRef]
- Moineau, G.; Minet, M.; Dubois, P.; Teyssie, P.; Senninger, T.; Jerome, R. Controlled radical polymerization of (meth)acrylates by ATRP with NiBr2(PPh3)2 as catalyst†. Macromolecules 1999, 32, 27–35. [Google Scholar] [CrossRef]
- Mayder, D.M.; Thompson, K.A.; Christopherson, C.J.; Paisley, N.R.; Hudsonet, Z.M. An efficient room-temperature synthesis of highly phosphorescent styrenic Pt(II) complexes and their polymerization by ATRP†. Polym. Chem. 2018, 9, 5418–5425. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Iron-catalyzed atom transfer radical polymerization. Polym. Chem. 2015, 6, 1660–1687. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, J.; Qiu, L.; Zhou, Y.; Xia, L.; Yan, F. Basic ionic liquids: A new type of ligand and catalyst for the AGET ATRP of methyl methacrylate†. Polym. Chem. 2012, 3, 2436–2443. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free photoinduced electron transfer-atom transfer radical polymerization (PET-ATRP) via a visible light organic photocatalyst†. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.T.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Georges, M.K.; Veregin, R.P.N.; Kazmaier, P.M.; Hamer, G.K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Krstina, J.; Moad, C.L.; Moad, G.; Rizzardo, E.; Berge, C.T.; Fryd, M. A new form of controlled growth free radical polymerization. Macromol. Symp. 1996, 111, 13–23. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Eracole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Zhu, Y.; Egap, E. PET-RAFT polymerization catalyzed by cadmium selenide quantum dots (QDs): Grafting-from QDs photocatalysts to make polymer nanocomposites†. Polym. Chem. 2020, 11, 1018–1024. [Google Scholar] [CrossRef]
- Wan, W.; Pan, C. One-pot synthesis of polymeric nanomaterials via RAFT dispersion polymerization induced self-assembly and re-organization. Polym. Chem. 2010, 1, 1475–1484. [Google Scholar] [CrossRef]
- Boyer, C.; Lacroix-Desmazes, P.; Robin, J.-J.; Boutevin, B. Reverse iodine transfer polymerization (RITP) of methyl methacrylate. Macromolecules 2006, 39, 4044–4053. [Google Scholar] [CrossRef]
- Kwak, Y.; Goto, A.; Fukuda, T.; Kobayashi, Y.; Yamago, S. A systematic study on activation processes in organotellurium-mediated living radical polymerizations of styrene, methyl methacrylate, methyl acrylate, and vinyl acetate. Macromolecules 2006, 39, 4671–4679. [Google Scholar] [CrossRef]
- Arita, T.; Kayama, Y.; Ohno, K.; Tsujii, Y.; Fukuda, T. High-pressure atom transfer radical polymerization of methyl methacrylate for well-defined ultrahigh molecular-weight polymers. Polymer 2008, 49, 2426–2429. [Google Scholar] [CrossRef]
- Rzayev, J.; Penelle, J. HP-RAFT: A free-radical polymerization technique for obtaining living polymers of ultrahigh molecular weights**. Angew. Chem. Int. Edit. 2004, 43, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Sugimoto, H.; Aida, T.; Inoue, S. Controlled synthesis of high molecular weight poly(methyl methacrylate) based on lewis acid-assisted high-speed living polymerization initiated with aluminum porphyrin. Macromolecules 1992, 25, 2280–2281. [Google Scholar] [CrossRef]
- Bai, Y.; He, J.; Zhang, Y. Ultra-high-molecular-weight polymers produced by the immortal phosphine-based catalyst system. Angew. Chem. 2018, 130, 17476–17480. [Google Scholar] [CrossRef]
- Ravve, A. Principles of Polymer Chemistry, 3rd ed.; Springer: Niles, IL, USA, 2012; pp. 1–240. [Google Scholar]
- Iwatsuici, S.; Kasahara, H.; Yamashit, Y. Polymerization of methylmethacrylate initiated by hydrogenation catalysts. Makromol. Chem. 1967, 104, 254–262. [Google Scholar] [CrossRef]
- Otsu, T.; Aoki, S.; Nishimura, M.; Yamaguchi, M.; Kusuki, Y. Initiation of vinyl polymerization by systems of activated metals and organic halides having various types of halogen bonds. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 835–837. [Google Scholar] [CrossRef]
- Otsu, T.; Yamaguchi, M. Metal-Containing Initiator Systems. IV. Polymerization of Methyl Methacrylate by Systems of Some Activated Metals and Organic Halides. J. Polym. Sci. Pol. Chem. 1968, 6, 3075–3085. [Google Scholar] [CrossRef]
- Otsu, T.; Aoki, S.; Nishimura, M.; Yamaguchi, M.; Kusuki, Y. Metal-containing initiator systems. V. radical and cationic polymerizations with initiator systems of reduced nickel and chlorosilanes. J. Polym. Sci. Part A 1 Polym. Chem. 1969, 7, 3269–3277. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of Palladium. Catalysis Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Spontoni, P.; Arrigo, R.; Su, D.; Prati, L. Nitrogen functionalized carbon nanostructures supported Pd and Au-Pd NPs as catalyst for alcohols oxidation. Catal. Today 2010, 157, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Yan, X.; Zou, P.; Wang, Y.; Dai, L. Large size Pd NPs loaded on TiO2 as efficient catalyst for the aerobic oxidation of alcohols to aldehydes. Catal. Commun. 2015, 58, 132–136. [Google Scholar] [CrossRef]
- Marulasiddeshwara, M.B.; Kumar, P.R. Synthesis of Pd(0) nanocatalyst using lignin in water for the Mizoroki-Heck reaction under solvent-free conditions. Int. J. Biol. Macromol. 2016, 83, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Paul, S.; Kundu, S.; Byström, E.; Irgum, K.; Almqvist, F. Organic polymeric resins embedded with Pd NPs: Newly designed, efficient and chemoselective catalyst for reduction of nitrobenzenes. Curr. Organocatal. 2017, 4, 48–61. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Z.; Luo, Y.; Zou, A.; Yao, Q.; Chen, X. Mesoporous carbon nitride supported Pd and Pd-Ni nanoparticles as highly efficient catalyst for catalytic hydrolysis of NH3BH3. ChemCatChem 2018, 10, 1620–1626. [Google Scholar] [CrossRef]
- Ganesan, M.; Freemantle, R.G.; Obare, S.O. Monodisperse thioether-stabilized Palladium nanoparticles: Synthesis, characterization, and reactivity. Chem. Mat. 2007, 19, 3464–3471. [Google Scholar] [CrossRef]
- Ohmes, E.; Kothe, G.; Naujok, A.; Zimmermann, H. Zur frage der assoziation von tri-p-biphenylyl-methyl eine ESR-spektroskopische untersuchung zum selwood-effekt. Ber. Bunsen. Ges. 1971, 75, 895–901. [Google Scholar] [CrossRef]
- Stradyn, Y.P.; Gavar, R.A.; Grin, V.K.; Hiller, S.A. Stability and rate of decay of anion radicals in the nitrofuran series. Theor. Exp. Chem. 1971, 4, 495–500. [Google Scholar] [CrossRef]
- Dai, S.; Wu, X.; Zhang, J.; Fu, Y.; Li, W. Coenzyme a-regulated Pd nanocatalysts for formic acid-mediated reduction of hexavalent chromium. Chem. Eng. J. 2018, 351, 959–966. [Google Scholar] [CrossRef]
- Campbell, D.; Pethrick, R.A.; White, J.R. Polymer Characterization: Physical Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–464. [Google Scholar]
- Borman, C.D.; Jackson, A.T.; Bunn, A.; Cutter, A.L.; Irvine, D.J. Evidence for the low thermal stability of poly(methyl methacrylate) polymer produced by atom transfer radical polymerisation. Polymer 2000, 41, 6015–6020. [Google Scholar] [CrossRef]
- Hatada, K.; Kitayama, T.; Ute, K.; Terawaki, Y.; Yanagida, T. End-group analysis of poly(methyl methacrylate) prepared with benzoyl peroxide by 750 MHz high-resolution 1H NMR spectroscopy. Macromolecules 1997, 30, 6754–6759. [Google Scholar] [CrossRef]
- Janzen, E.G.; Blackburn, B.J. Detection and identification of short-lived free radicals by electron spin resonance trapping techniques (spin trapping). Photolysis of organolead, -tin, and -mercury compounds. J. Am. Chem. Soc. 1969, 91, 4481–4490. [Google Scholar] [CrossRef]
- Kamachi, M.; Kuwae, Y.; Nozakura, S. Spin trapping study on addition reaction of organic radicals to methyl methacrylate and methyl tiglate. Polym. Bull. 1981, 6, 143–146. [Google Scholar] [CrossRef]
- Fischer, H. The persistent radical effect: A principle for selective radical reactions and living radical polymerization. Chem. Rev. 2001, 101, 3581–3610. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Robert, W.; Diego, M.; Manfred, H.; Daniel, F.; Eugenio, T.; Silvia, G. Pt(II) and Pd(II) pyrrolidine-dithiocarbamates investigated by XPS. Surf. Sci. Spectra 2011, 18, 82–95. [Google Scholar]





| Entry | Mono. | Init. | Mono./Init./Pd NP (Molar Ratio) | T (°C) | Time (h) | Conv. (%) | Mn (Da) | Mw (Da) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MMA | CCl4 | 1.31 × 104:76:1 | 70 | 6.0 | 86.6 | 8.84 × 105 | 1.59 × 106 | 1.80 |
| 2 | MMA | BPA | 1.31 × 104:76:1 | 70 | 3.1 | 32.5 | 2.56 × 105 | 5.66 × 105 | 2.21 |
| 3 | MMA | EBP | 1.31 × 104:76:1 | 80 | 5.9 | 38.3 | 7.96 × 105 | 1.72 × 106 | 2.16 |
| 4 | MMA | EBiB | 1.31 × 104:76:1 | 70 | 6.0 | 93.0 | 1.32 × 106 | 2.61 × 106 | 1.98 |
| 5 | MMA | EBiB | 1.05 × 105:76:1 | 70 | 24.0 | 82.8 | 4.65 × 106 | 8.08 × 106 | 1.73 |
| 6 | MMA | EBiB | 1.05 × 105:76:1 | 80 | 24.0 | 89.0 | 3.96 × 106 | 7.25 × 106 | 1.83 |
| 7 | MMA | EBiB | 1.05 × 105:76:1 | 90 | 24.0 | 91.2 | 3.00 × 106 | 6.06 × 106 | 2.02 |
| 8 | MA | EBiB | 1.56 × 104:76:1 | 70 | 3.0 | 91.6 | 1.32 × 106 | 2.72 × 106 | 2.06 |
| 9 | BA | EBiB | 9.90 × 103:76:1 | 70 | 3.0 | 83.0 | 5.77 × 105 | 1.15 × 106 | 1.99 |
| 10 | VAc 2 | EBiB | 9.71 × 102:4.8:1 | 80 | 24.0 | 43.7 | 1.66 × 104 | 3.86 × 104 | 2.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Xu, L.; Cui, X.; Lv, J.; Zhang, P.; Tang, H. Facile Synthesis of Ultrahigh Molecular Weight Poly(Methyl Methacrylate) by Organic Halides in the Presence of Palladium Nanoparticles. Polymers 2020, 12, 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112747
Yuan M, Xu L, Cui X, Lv J, Zhang P, Tang H. Facile Synthesis of Ultrahigh Molecular Weight Poly(Methyl Methacrylate) by Organic Halides in the Presence of Palladium Nanoparticles. Polymers. 2020; 12(11):2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112747
Chicago/Turabian StyleYuan, Ming, Lili Xu, Xuetao Cui, Jiaxing Lv, Panpan Zhang, and Huadong Tang. 2020. "Facile Synthesis of Ultrahigh Molecular Weight Poly(Methyl Methacrylate) by Organic Halides in the Presence of Palladium Nanoparticles" Polymers 12, no. 11: 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112747