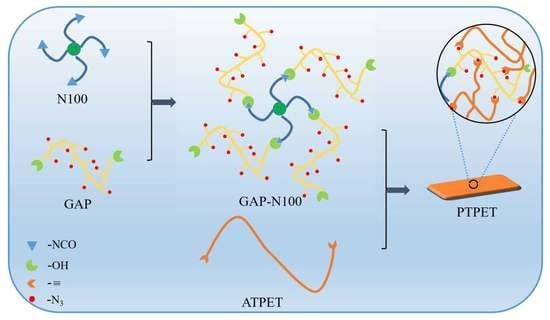
The Effect of Glycidyl Azide Polymer Grafted Tetrafunctional Isocyanate on Polytriazole Polyethylene Oxide-Tetrahydrofuran Elastomer and its Propellant Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of N100-g-GAP
2.3. Preparation of Polytriazole Polyethylene Oxide-Tetrahydrofuran Elastomer
2.4. Application of N100-g-GAP in PTPET Composite Propellants
2.5. Measurements and Analysis
3. Results and Discussion
3.1. IR and GPC Analysis of N100-g-GAP
3.2. Thermal Stability Analysis of PTPET Elastomer
3.3. Dynamical Mechanical Analysis of the PTPET Elastomer
3.4. Swelling Analysis of PTPET Prepared Elastomer
3.5. Tensile Behaviors of PTPET Elastomer
3.6. Thermal Stability Analyses of Propellants
3.7. Dynamical Mechanical Analyses of Propellants
3.8. Mechanical Properties of Propellants
3.9. SEM Results of Propellants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohn, M.A.; Elsner, P. Aging of the binders GAP-N100 and HTPB-IPDI investigated by torsion-DMA. Propellants Explos. Pyrotech. 1999, 24, 199–205. [Google Scholar] [CrossRef]
- Reshmi, S.K.; Vijayalakshmi, K.P.; Thomas, D.; Arunan, E.; Nair, C.P.R. Glycidyl Azide Polymer Crosslinked Through Triazoles by Click Chemistry: Curing, Mechanical and Thermal Properties. Propellants Explos. Pyrotech. 2013, 38, 525–532. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem.-Int. Ed. 2001, 40, 2004. [Google Scholar] [CrossRef]
- Demko, Z.P.; Sharpless, K.B. A click chemistry approach to tetrazoles by Huisgen 1,3-dipolar cycloaddition: Synthesis of 5-sulfonyl tetrazoles from azides and sulfonyl cyanides. Angew. Chem.-Int. Ed. 2002, 41, 2110–2113. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “Clicking” by CuAAC reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Min, B.S.; Park, Y.C.; Yoo, J.C. A Study on the Triazole Crosslinked Polymeric Binder Based on Glycidyl Azide Polymer and Dipolarophile Curing Agents. Propellants Explos. Pyrotech. 2012, 37, 59–68. [Google Scholar] [CrossRef]
- Sonawane, S.; Anniyappan, M.; Athar, J.; Singh, A.; Talawar, M.B.; Sinha, R.K.; Banerjee, S.; Sikder, A.K. Isocyanate-Free Curing of Glycidyl Azide Polymer with Bis-propargylhydroquinone. Propellants Explos. Pyrotech. 2017, 42, 386–393. [Google Scholar] [CrossRef]
- Hagen, T.H.; Jensen, T.L.; Unneberg, E.; Stenstrom, Y.H.; Kristensen, T.E. Curing of Glycidyl Azide Polymer (GAP) Diol Using Isocyanate, Isocyanate-Free, Synchronous Dual, and Sequential Dual Curing Systems. Propellants Explos. Pyrotech. 2015, 40, 275–284. [Google Scholar] [CrossRef]
- Liu, F.-B.; Zhang, X.-L.; Jiang, W.-S.; Yu, F.-Y.; Deng, J.-R. Study on the Curing System of Polytriazole Adhesive for Composite Solid Propellant. Propellants Explos. Pyrotech. 2018, 43, 371–378. [Google Scholar] [CrossRef]
- Zou, Y. Study on the Polyether Elastomer and Its Performances in Composite Solid Propellant; Beijing Institute of Technology: Beijing, China, 2018. [Google Scholar]
- Qu, Z.; Zhai, J.; Yang, R. Comparison between properties of polyether polytriazole elastomers and polyether polyurethane elastomers. Polym. Adv. Technol. 2014, 25, 314–321. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, R.; Zhai, J. Polytriazole polyether elastomers with widely tunable mechanical properties: The role of network structure and crystallization behavior. J. Appl. Polym. Sci. 2017, 134, 45298. [Google Scholar] [CrossRef]
- Everaers, R.; Grosberg, A.Y.; Rubinstein, M.; Rosa, A. Flory theory of randomly branched polymers. Soft Matter 2017, 13, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.-f.; Shi, L.; Yang, W.; Yao, W.-S. Study on the Synthesis and Interfacial Interaction Performance of Novel Dodecylamine-Based Bonding Agents Used for Composite Solid Propellants. Propellants Explos. Pyrotech. 2015, 40, 50–59. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Li, G.; Luo, Y. Effect of Bonding Agent on the Mechanical Properties of GAP High-Energy Propellant. Propellants Explos. Pyrotech. 2017, 42, 394–400. [Google Scholar] [CrossRef]
- Landsem, E.; Jensen, T.L.; Hansen, F.K.; Unneberg, E.; Kristensen, T.E. Neutral Polymeric Bonding Agents (NPBA) and Their Use in Smokeless Composite Rocket Propellants Based on HMX-GAP-BuNENA. Propellants Explos. Pyrotech. 2012, 37, 581–591. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, M.-E.; Tan, X.-L.; Peng, J.; Deng, J.-R. Synthesis and Application of Borate Ester Bonding Agents for a Four-Component Hydroxy-Terminated Polybutadiene Propellant. Propellants Explos. Pyrotech. 2015, 40, 831–837. [Google Scholar] [CrossRef]
- Sun, Q.; Sang, C.; Wang, Z.; Luo, Y. The study of mechanical and creep properties of glycidyl azide polyol energetic thermoplastic elastomer binder with bonding group with RDX and its interface reinforcement mechanism. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bristow, G.M.; Watson, W.F. Cohesive energy densities of polymers. Part 1.—Cohesive energy densities of rubbers by swelling measurements. Trans. Faraday Soc. 1958, 54, 1731–1741. [Google Scholar] [CrossRef]
- Yarmohammadi, M.; Komeili, S.; Shahidzadeh, M. Studying Crosslinker Chemical Structure Effect on the Tuning Properties of HTPB-Based Polyurethane. Propellants Explos. Pyrotech. 2018, 43, 156–161. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kang, M.-S.; Knowles, J.C.; Gong, M.-S. Synthesis of bio-based thermoplastic polyurethane elastomers containing isosorbide and polycarbonate diol and their biocompatible properties. J. Biomater. Appl. 2015, 30, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.-Y.; Lu, Y.-L.; Xiao, D.-L.; Wu, S.-Z.; Zhang, L.-Q. Thermoplastic Ternary Hybrids of Polyurethane, Hindered Phenol and Hindered Amine with Selective Two-Phase Dispersion. Macromol. Mater. Eng. 2009, 294, 345–351. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Peng, S.W.; Zhao, X.J.; Wang, X.H.; Dong, L.S. FTIR study of poly(propylene carbonate)/bisphenol A blends. Polym. Int. 2004, 53, 2092–2098. [Google Scholar] [CrossRef]
- Vernooij, E.; Kettenes-van den Bosch, J.J.; Crommelin, D.J.A. Fourier transform infrared spectroscopic determination of the hydrolysis of poly(ethylene glycol)-phosphatidylethanolamine-containing liposomes. Langmuir 2002, 18, 3466–3470. [Google Scholar] [CrossRef]
- Kennedy, J.M.; Edie, D.D.; Banerjee, A.; Cano, R.J. Characterization of interfacial bonds strength by dynamic analysis. J. Compos. Mater. 1992, 26, 869–882. [Google Scholar] [CrossRef]










| PTPET Samples | χ1 | qv | ν2m | ρ/(g·cm−3) | MC/(g·mol−1) | N0/(mmol·cm−3) |
|---|---|---|---|---|---|---|
| GAP | 0.340 | 5.439 | 0.184 | 1.075 | 7405 | 0.150 |
| N100-g-GAP | 0.340 | 3.992 | 0.251 | 1.079 | 3510 | 0.307 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Tang, W.; Li, Y.; He, J.; Guo, X.; Yang, R. The Effect of Glycidyl Azide Polymer Grafted Tetrafunctional Isocyanate on Polytriazole Polyethylene Oxide-Tetrahydrofuran Elastomer and its Propellant Properties. Polymers 2020, 12, 278. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020278
Hu J, Tang W, Li Y, He J, Guo X, Yang R. The Effect of Glycidyl Azide Polymer Grafted Tetrafunctional Isocyanate on Polytriazole Polyethylene Oxide-Tetrahydrofuran Elastomer and its Propellant Properties. Polymers. 2020; 12(2):278. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020278
Chicago/Turabian StyleHu, Jinghui, Weiqiang Tang, Yonghui Li, Jiyu He, Xiaoyan Guo, and Rongjie Yang. 2020. "The Effect of Glycidyl Azide Polymer Grafted Tetrafunctional Isocyanate on Polytriazole Polyethylene Oxide-Tetrahydrofuran Elastomer and its Propellant Properties" Polymers 12, no. 2: 278. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020278