A Feasible Method Applied to One-Bath Process of Wool/Acrylic Blended Fabrics with Novel Heterocyclic Reactive Dyes and Application Properties of Dyed Textiles
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentation
2.2. Preparation
2.3. Dyeing and Measure Methods
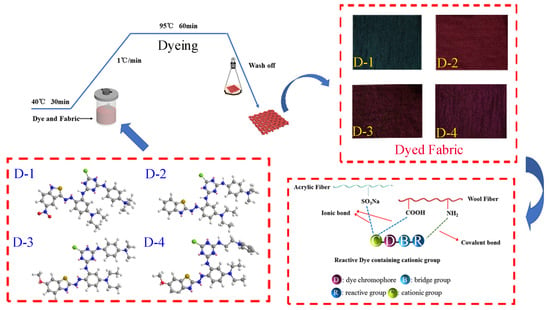
2.3.1. One-Bath One-Step Procedure
2.3.2. Dye Exhaustion
2.3.3. The Color Strength (K/S) on the Dyed Fabrics
2.3.4. Fabric Strength Test
2.3.5. Levelling Test
2.3.6. Fastness Testing
2.3.7. Anti-Ultraviolet Testing
2.3.8. Antibacterial Testing
3. Results and Discussion
3.1. Spectral Characterization
3.2. Dyeing Properties of the Four Reactive Dyes
3.3. Effect of pH Value
3.4. Effect of Temperature
3.5. Dyeing Heat-Rate Curve
3.6. Build-Up and Homochromaticity of Different Dyed Fabrics
3.7. Fabric Strength Test
3.8. Levelling Properties
3.9. Fastness Properties of the Dyed Fabrics
3.10. Anti-Ultraviolet Properties of Dyed Fabric
3.11. Antibacterial Properties of Dyed Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, W.; Cheng, Y.; Zhang, R.; Qin, C.; Chen, G. Preparing Fluorescent Wool/Acrylic Blends with a Hemicyanine Reactive Cationic Dye. Fibers Polym. 2016, 17, 15–20. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; El-Zawahry, M.M.; Ahmed, N.S.E. One-bath union dyeing of a modified wool/acrylic blend with acid and reactive dyes. Color. Technol. 2011, 127, 28–38. [Google Scholar] [CrossRef]
- Lee, J.J.; Han, N.K.; Lee, W.J.; Choi, J.H.; Kim, J.P. One-bath dyeing of a polyester/cotton blend with reactive disperse dyes from 2-hydroxypyrid-6-one derivatives. Color. Technol. 2003, 119, 134–139. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Gharanjig, K.; Arami, M. Study on dyeing and fastness properties of wool-polyester blend fabrics using novel mono azo-naphthalimide dyes. J. Text. Inst. 2014, 105, 52–58. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Dou, H.; Pei, L. Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System. Polymers 2018, 10, 1158. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Lin, F.; Chen, X.; Ye, W.; Li, X.; Zeng, H.; Van der Bruggen, B. Sustainable Management of Textile Wastewater: A Hybrid Tight Ultrafiltration/Bipolar-Membrane Electrodialysis Process for Resource Recovery and Zero Liquid Discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. [Google Scholar] [CrossRef]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and Dyes: Developments and Applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Zhao, T. One-Bath Union Dyeing of Wool/Acrylic Blend Fabric with Cationic Reactive Dyes Based on Azobenzene. Fibers Polym. 2018, 19, 331–339. [Google Scholar] [CrossRef]
- Zhao, T.; Gehui, W. Dyeing and Antimicrobial Properties of Antimicrobial Cationic Reactive Dye on Different Kind of Fibers. J. Donghua Univ. 2010, 36, 645–648. [Google Scholar]
- Xiao, H.; Zhao, T.; Li, C.H.; Li, M.Y. Eco-friendly approaches for dyeing multiple type of fabrics with cationic reactive dyes. J. Clean Prod. 2017, 165, 1499–1507. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, M.; Zhao, T. Dyeing performance of reactive cationic dyes. Dye. Finish. 2016, 42, 22–25. [Google Scholar]
- Xiao, H.; Li, C.; Wang, P.; Zhao, T. A feasible approach for enhancing union dyeing of wool/acrylic blend fabrics with heterobifunctional cationic reactive dyes. Text. Res. J. 2019, 89, 5085–5095. [Google Scholar] [CrossRef]
- Xie, K.L.; Hou, A.Q. One-bath dyeing of wool/acrylic blends with reactive cationic dyes based on monofluorotriazine. Color. Technol. 2004, 120, 307–310. [Google Scholar] [CrossRef]
- Wang, G.W.; Zheng, C.L.; Sun, J. Synthesis and salt-free dyeing characteristics of cationic reactive dyes containing polyetheramine segments. Color. Technol. 2016, 132, 344–349. [Google Scholar] [CrossRef]
- Javadi, M.S.; Mokhtari, J. Synthesis and Evaluation of Technical Properties of Novel Cationic Mono-s-chloro Triazinyl (MCT) Reactive Dyes on Cotton. J. Chin. Chem. Soc. 2012, 59, 793–801. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Taylor, J.A. Dyeing of nylon with reactive dyes. Part 3: Cationic reactive dyes for nylon. Dyes Pigment. 2008, 76, 610–623. [Google Scholar] [CrossRef]
- Liu, J.; Sun, G. The synthesis of novel cationic anthraquinone dyes with high potent antimicrobial activity. Dyes Pigment. 2008, 77, 380–386. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Taylor, J.A. Synthesis and evaluation of a novel blue cationic reactive dye for modified nylon 6.6 ‘Tactel Coloursafe’. Color. Technol. 2011, 127, 227–234. [Google Scholar] [CrossRef]
- Shah, S.S.; Ahmad, R.; Shah, S.W.H.; Asif, K.M.; Naeem, K. Synthesis of cationic hemicyanine dyes and their interactions with ionic surfactants. Colloids Surf. A 1998, 137, 301–305. [Google Scholar] [CrossRef]
- Şener, N.; Gür, M.; Çavuş, M.S.; Zurnaci, M. Synthesis, Characterization, and Theoretical Calculation of New Azo Dyes Derived from [1,5]Pyrimidinene Having Solvatochromic Properties. J. Heterocyclic. Chem. 2019, 56, 1101–1110. [Google Scholar] [CrossRef]
- Ho, Y.W.; Yao, W.H. Synthesis and properties of heterocyclic monoazo dyes derived from 3-cyano-4-trifluoromethyl-6-substituted-2(1H)-pyridinethiones. Dyes Pigment. 2006, 70, 60–69. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, Y.H.; Tao, T.; Qian, H.F.; Huang, W. Structural and spectral comparisons between isomeric benzisothiazole and benzothiazole based aromatic heterocyclic dyes. J. Mol. Struct. 2015, 1095, 42–50. [Google Scholar] [CrossRef]
- Gao, A.; Zhang, H.; Hou, A.; Xie, K. Dyeing properties of the disperse dyes containing cyano group based on benzisothiazole for polyester fabrics under alkali condition. Fibers Polym. 2017, 18, 1956–1961. [Google Scholar] [CrossRef]
- Qian, H.F.; Wang, Y.G.; Chen, X.C.; Ruan, W.G.; Huang, W. Structural and spectral characterizations of C.I. Disperse Blue 148 having a new crystalline form. Dyes Pigment. 2013, 99, 489–495. [Google Scholar] [CrossRef]
- Elgazwy, A. The chemistry of isothiazoles. Tetrahedron 2003, 59, 7445–7463. [Google Scholar] [CrossRef]
- Maradiya, H.R.; Patel, V.S. Dyeing performance of disperse dyes based on 2-aminothiazole for cellulose triacetate and nylon fibers. Fibers Polym. 2002, 3, 43–48. [Google Scholar] [CrossRef]
- Yen, M. A facile syntheses and absorption characteristics of some monoazo dyes in bis-heterocyclic aromatic systemspart II: Syntheses of 4-(p-substituted) phenyl-2-(2-pyrido-5-yl and 5-pyrazolo-4-yl) azo-thiazole derivatives. Dyes Pigment. 2004, 63, 1–9. [Google Scholar] [CrossRef]
- Tao, T.; Xu, F.; Chen, X.C.; Liu, Q.Q.; Huang, W.; You, X.Z. Comparisons between azo dyes and Schiff bases having the same benzothiazole/phenol skeleton: Syntheses, crystal structures and spectroscopic properties. Dyes Pigment. 2012, 92, 916–922. [Google Scholar] [CrossRef]
- Pu, S.; Liu, W.; Liu, G. The photochromism of unsymmetrical diarylethene isomers with an electron-withdrawing cyano substituent. Dyes Pigment. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Riva, A.; Algaba, I.; Pepio, M.; Prieto, R. Modeling the Effects of Color on the UV Protection Provided by Cotton Woven Fabrics Dyed with Azo Dyestuffs. Ind. Eng. Chem. Res. 2009, 48, 9817–9822. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, application and antibacterial activity of new reactive dyes based on thiazole moiety. Pigm. Resin Technol. 2018, 47, 246–254. [Google Scholar] [CrossRef]
- Silva, M.G.d.; Barros, M.A.S.D.d.; Almeida, R.T.R.d.; Pilau, E.J.; Pinto, E.; Soares, G.; Santos, J.G. Cleaner production of antimicrobial and anti-UV cotton materials through dyeing with eucalyptus leaves extract. J. Clean Prod. 2018, 199, 807–816. [Google Scholar] [CrossRef]
- Jeevarathinam, A.S.; Pai, N.; Huang, K.; Hariri, A.; Wang, J.; Bai, Y.; Wang, L.; Hancock, T.; Keys, S.; Penny, W.; et al. A cellulose-based photoacoustic sensor to measure heparin concentration and activity in human blood samples. Biosens. Bioelectron. 2019, 126, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aktek, T.; Millat, A.K.M.M. Salt Free Dyeing of Cotton Fiber—A Critical Review. Int. J. Text. Sci. 2017, 2, 21–33. [Google Scholar]
- Srikulkit, K.; Santifuengkul, P. Salt-free dyeing of cotton cellulose with a model cationic reactive dye. J. Soc. Dyers Colour. 2000, 116, 398–402. [Google Scholar] [CrossRef]
- Farouk, R.; Gaffer, H.E. Simultaneous dyeing and antibacterial finishing for cotton cellulose using a new reactive dye. Carbohydr. Polym. 2013, 97, 138–142. [Google Scholar] [CrossRef]
- Zheng, C.; Yuan, A.; Wang, H.; Sun, J. Dyeing properties of novel electrolyte-free reactive dyes on cotton fibre. Color. Technol. 2012, 128, 204–207. [Google Scholar] [CrossRef]
- Xie, K.; Gao, A.; Li, M.; Wang, X. Printing properties of the red reactive dyes with different number sulfonate groups on cotton fabric. Carbohydr. Polym. 2014, 101, 666–670. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Youssef, Y.A.; Ahmed, N.S.E.; Mousa, A.A. The use of sodium edate in dyeing: II. Union dyeing of cotton/wool blend with hetero bi-functional reactive dyes. Dyes Pigment. 2007, 72, 57–65. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Peng, Y.X.; Geng, J.; Qian, H.F.; Huang, W. Post-modification of 2-formylthiophene based heterocyclic azo dyes. Dyes Pigment. 2016, 133, 143–152. [Google Scholar] [CrossRef]
- Hamidian, H.; Zahedian, N.; Ghazanfari, D.; Fozooni, S. Synthesis and Evaluation of Changes Induced by Solvent and Substituent in Electronic Absorption Spectra of New Azo Disperse Dyes Containig Barbiturate Ring. J. Spectrosc. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Yazdanbakhsh, M.R.; Farahnak, L. Synthesis and evaluation of changes induced by solvent and substituent in electronic absorption spectra of some azo disperse dyes. Spectrochim. Acta A 2012, 89, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Kuster, S.; Yum, J.H.; Moon, S.J.; Nazeeruddin, M.K.; Grätzel, M.; Nüesch, F. Molecular Design of Unsymmetrical Squaraine Dyes for High Efficiency Conversion of Low Energy Photons into Electrons Using TiO2 Nanocrystalline Films. Adv. Funct. Mater. 2009, 19, 2720–2727. [Google Scholar] [CrossRef]
- Zhan, Y.Z.; Zhao, X.; Wang, W. Synthesis and application of phthalimide disperse dyes. Fibers Polym. 2017, 18, 2278–2286. [Google Scholar] [CrossRef]
- Brédas, J.L. Organic Electronics: Does a Plot of the HOMO–LUMO Wave Functions Provide Useful Information? Chem. Mater. 2017, 29, 477–478. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Cai, J.; Ren, J.; Cui, Z.; Chen, W. Quinoidal bithiophene as disperse dye: Substituent effect on dyeing performance. Dyes Pigment. 2018, 151, 363–371. [Google Scholar] [CrossRef]
- El Gabry, L.K. Effect of mineral acids on the properties of acrylic fabrics. Color. Technol. 2004, 120, 236–240. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.Y.; Cheng, X.W.; Tang, R.C.; Qiao, Y.F. Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber. Polymers 2019, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Liu, X.; Rex Brady, P. One-bath union dyeing of wool/polytrimethylene terephthalate blends. Color. Technol. 2008, 124, 204–210. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sun, G. Durable antimicrobial finishing of nylon fabrics with acid dyes and a quaternary ammonium salt. Text. Res. J. 2001, 71, 318–323. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles—A Review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef] [PubMed]















| Dye | λDMF (nm) | λMethanol (nm) | λIsopropanol (nm) | ε (L mol−1 cm−1) | Color | λonset (nm) | (ev) |
|---|---|---|---|---|---|---|---|
| D-1 | 633.0 | 623.0 | 621.0 | 26634 | greenish blue | 712.0 | 1.74 |
| D-2 | 536.0 | 535.0 | 532.0 | 34435 | fuchsia | 614.0 | 2.02 |
| D-3 | 535.0 | 534.0 | 532.0 | 55247 | fuchsia | 626.0 | 1.98 |
| D-4 | 542.0 | 539.0 | 536.0 | 43379 | fuchsia | 624.0 | 1.99 |
| Wool Fabric | Acrylic Fabric | Wool/Acrylic Blended Fabric | ||
|---|---|---|---|---|
| D-1 | E% | 97.68% | 99.94% | 97.85% |
| K/S | 5.33 | 5.05 | 6.16 | |
| D-2 | E% | 99.86% | 99.75% | 99.44% |
| K/S | 16.52 | 9.61 | 11.18 | |
| D-3 | E% | 99.91% | 99.43% | 99.97% |
| K/S | 29.18 | 12.77 | 12.29 | |
| D-4 | E% | 99.89% | 98.71% | 99.91% |
| K/S | 28.85 | 6.30 | 9.10 | |
| Dyes | Fabrics | L * | a * | b * | K/S | k |
|---|---|---|---|---|---|---|
| D-1 | wool | 39.40 | −16.23 | −7.43 | 5.33 | 1.06 |
| acrylic | 41.49 | −15.82 | −7.61 | 5.05 | ||
| D-2 | wool | 28.24 | 38.15 | −0.97 | 16.52 | 1.72 |
| acrylic | 32.41 | 34.80 | −6.62 | 9.61 | ||
| D-3 | wool | 20.71 | 24.52 | −3.82 | 29.18 | 2.29 |
| acrylic | 28.64 | 31.32 | −10.21 | 12.77 | ||
| D-4 | wool | 22.47 | 32.29 | −9.20 | 28.85 | 4.58 |
| acrylic | 28.48 | 19.64 | −4.54 | 6.30 |
| Wool Fabric | Acrylic Fabric | Wool/Acrylic Fabric | |
|---|---|---|---|
| Untreated fabric | 219.1 N | 331.8 N | 379.3 N |
| Dyed fabric with D-1 | 213.5 N | 325.3 N | 373.1 N |
| Dyed fabrics with D-2 | 212.8 N | 324.6 N | 372.2 N |
| Dyed fabrics with D-3 | 212.5 N | 324.7 N | 372.5 N |
| Dyed fabrics with D-4 | 214.6 N | 325.5 N | 373.4 N |
| Samples | Digital Pictures for Blended Fabric (1%owf) | Rubbing Fastness | Washing Fastness | Light Fastness | |||
|---|---|---|---|---|---|---|---|
| Dry | Wet | SC | SW | SA | |||
| D-1 |  | 5 | 4 | 4 | 4 | 4–5 | 4 |
| D-2 |  | 5 | 4 | 4 | 4 | 4–5 | 4–5 |
| D-3 |  | 4–5 | 3–4 | 4 | 4 | 4–5 | 4–5 |
| D-4 |  | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 4–5 |
| Types | Undyed Fabric | D-1 | D-2 | D-3 | D-4 |
|---|---|---|---|---|---|
| Antibacterial rate (%) | 0 | 88.09 | 91.23 | 94.50 | 90.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, X.; Guo, C.; Zhao, T.; Li, W. A Feasible Method Applied to One-Bath Process of Wool/Acrylic Blended Fabrics with Novel Heterocyclic Reactive Dyes and Application Properties of Dyed Textiles. Polymers 2020, 12, 285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020285
Wang M, Wang X, Guo C, Zhao T, Li W. A Feasible Method Applied to One-Bath Process of Wool/Acrylic Blended Fabrics with Novel Heterocyclic Reactive Dyes and Application Properties of Dyed Textiles. Polymers. 2020; 12(2):285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020285
Chicago/Turabian StyleWang, Meihui, Xianfeng Wang, Chong Guo, Tao Zhao, and Wenyao Li. 2020. "A Feasible Method Applied to One-Bath Process of Wool/Acrylic Blended Fabrics with Novel Heterocyclic Reactive Dyes and Application Properties of Dyed Textiles" Polymers 12, no. 2: 285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020285