Effect of Phenolic Resin Oligomer Motion Ability on Energy Dissipation of Poly (Butyl Methacrylate)/Phenolic Resins Composites
Abstract
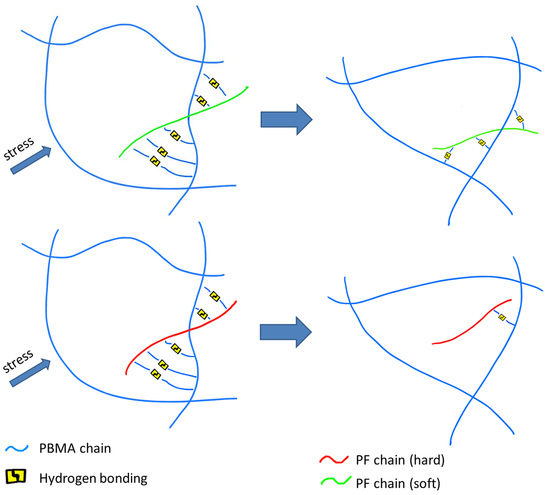
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of PBMA via Emulsion Polymerization
2.3. Preparation of PBMA/PR Composites via Solvent Blending
2.4. Characterization
3. Results and Discussion
3.1. Structure Characterization of Blends and PBMA
3.2. Glass Transition of PBMA/PR Composites
3.3. Molecular Dynamics of PBMA/PR Composites
3.4. Intermolecular Interaction between PBMA and PR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kishi, H.; Kuwata, M.; Matsuda, S.; Asami, T.; Murakami, A. Damping properties of thermoplastic-elastomer interleaved carbon fiber-reinforced epoxy composites. Compos. Sci. Technol. 2004, 64, 2517–2523. [Google Scholar] [CrossRef]
- Fan, R.; Meng, G.; Yang, J.; He, C. Experimental study of the effect of viscoelastic damping materials on noise and vibration reduction within railway vehicles. J. Sound Vib. 2009, 319, 58–76. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Z.P.; Liu, X.M.; Zhang, J.C. Preparation and Damping property Study of Styrene-Acrylic IPN/Mt Nano-composite Material. Adv. Mater. Res. 2011, 284–286, 382–386. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, C.; Hu, J.; He, X.; Zhang, R. Damping Analysis of Some Inorganic Particles on Poly(butyl-methacrylate). Materials 2018, 11, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andjelkovic, D.D.; Lu, Y.; Kessler, M.R.; Larock, R.C. Novel Rubbers from the Cationic Copolymerization of Soybean Oils and Dicyclopentadiene, 2–Mechanical and Damping Properties. Macromol. Mater. Eng. 2009, 294, 472–483. [Google Scholar] [CrossRef]
- He, X.R.; Yu, H.; Zhang, R.; Yang, C.H. Enhance Slower Relaxation Process of Poly(ethyl acrylate) through Internal Plasticization. Int. Polym. Process. 2014, 29, 419–424. [Google Scholar] [CrossRef]
- Rui, Z.; He, X.; Hui, Y. Why tanδ of poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar]
- Zhang, R.; He, X.; Huang, G. Dynamics of Poly (butyl acrylate) and Poly (ethyl acrylate) with internal double bonds. J. Polym. Res. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, X.; Zhu, J.; Hu, H.; Song, M.; Wu, S. Experimental and molecular dynamics simulation study on the damping mechanism of C5 petroleum resin/chlorinated butyl rubber composites. J. Mater. Sci. 2019, 54, 3960–3974. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Wang, X.; He, X.; Lei, H. Molecular dynamics in chlorinated butyl rubber containing organophilic montmorillonite nanoparticles. J. Polym. Res. 2011, 18, 2213–2220. [Google Scholar] [CrossRef]
- Ping, J.; Yang, C.; He, X.; Rodrigues, A.M.; Rui, Z. Viscoelastic changes in chlorinated butyl rubber modified with graphene oxide. Iran. Polym. J. 2017, 26, 861–870. [Google Scholar]
- Liao, K.H.; Aoyama, S.; Abdala, A.A.; Macosko, C. Does Graphene Change T g of Nanocomposites? Macromolecules 2014, 47, 8311–8319. [Google Scholar] [CrossRef]
- Wu, C. Effects of a hindered phenol compound on the dynamic mechanical properties of chlorinated polyethylene, acrylic rubber, and their blend. J. Appl. Polym. Sci. 2010, 80, 2468–2473. [Google Scholar] [CrossRef]
- Shi, G.; Yin, X.; Wu, G. Thermodynamic phase analysis of acrylic polymer/hindered phenol hybrids: Effects of hydrogen bonding strength. Polymer 2018, 153, 317–324. [Google Scholar] [CrossRef]
- Yin, X.; Liu, C.; Lin, Y.; Guan, A.; Wu, G. Influence of hydrogen bonding interaction on the damping properties of poly(n-butyl methacrylate)/small molecule hybrids. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Fuhai, C.; Jincheng, W. Preparation and characterization of hyperbranched polymer modified montmorillonite/chlorinated butyl rubber damping composites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Sheng, Z.; Yang, S.; Wang, J.; Lu, Y.; Tang, K.; Song, S. Preparation and Properties Analysis of Chlorinated Butyl Rubber (CIIR)/Organic Diatomite Damping Composites. Materials 2018, 11, 2172. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Zhao, C.; Xu, L.; Zhang, C. Effects of Chemical Structure of Phenolic Resin on Damping Properties of Acrylate Rubber-Based Blends. J. Macromol. Sci. Part B 2015, 54, 177–189. [Google Scholar] [CrossRef]
- Wu, C.; Wei, C.; Guo, W.; Wu, C. Dynamic mechanical properties of acrylic rubber blended with phenolic resin. J. Appl. Polym. Sci. 2008, 109, 2065–2070. [Google Scholar] [CrossRef]
- He, X.; Huang, G.; Zhou, H.; Jiang, L.; Zhao, X.; Zhang, J.; Wang, J. Investigation on the damping behavior of ciir/PMAc blends. Acta Polym. Sin. 2005, 108–112. [Google Scholar]
- Gurunath, P.V.; Bijwe, J. Friction and wear studies on brake-pad materials based on newly developed resin. Wear 2007, 263, 1212–1219. [Google Scholar] [CrossRef]
- Dapeng, Z.; Hong, F. Mechanical and High-Temperature Properties of Glass Fibers Reinforced Phenolic Composites. J. Reinf. Plast. Compos. 2008, 27, 1449–1460. [Google Scholar] [CrossRef]
- Qu, L.; Huang, G.; Wu, J.; Tang, Z. Damping mechanism of chlorobutyl rubber and phenolic resin vulcanized blends. J. Mater. Sci. 2007, 42, 7256–7262. [Google Scholar] [CrossRef]
- Rui, Z.; He, X.; Hui, Y.; Chen, K. Detecting the Rouse and Sub-Rouse Modes in Poly(Butyl Acrylate) and Poly(Ethyl Acrylate) Through Two-Dimensional Dynamic Mechanical Spectra. J. Macromol. Sci. Part B 2014, 53, 1642–1653. [Google Scholar]
- Lei, Z.; Xing, W.; Wu, J.; Huang, G.; Wang, X.; Zhao, L. The proper glass transition temperature of amorphous polymers on dynamic mechanical spectra. J. Therm. Anal. Calorim. 2014, 116, 447–453. [Google Scholar] [CrossRef]
- Zhang, F.; He, G.; Xu, K.; Wu, H.; Guo, S.; Zhang, C. Damping mechanism and different modes of molecular motion through the glass transition of chlorinated butyl rubber and petroleum resin blends. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Li, Z.; Lu, X.; Tao, G.; Guo, J.; Jiang, H. Damping elastomer with broad temperature range based on irregular networks formed by end-linking of hydroxyl-terminated poly(dimethylsiloxane). Polym. Eng. Sci. 2016, 56, 97–102. [Google Scholar] [CrossRef]
- Xia, L.; Li, C.; Zhang, X.; Wang, J.; Wu, H.; Guo, S. Effect of chain length of polyisobutylene oligomers on the molecular motion modes of butyl rubber: Damping property. Polymer 2018, 141, 70–78. [Google Scholar] [CrossRef]
- Radmard, B.; Dadmun, M.D. The accessibility of functional groups to intermolecular hydrogen bonding in polymer blends containing a liquid crystalline polymer. Polymer 2001, 42, 1591–1600. [Google Scholar] [CrossRef]
- Romeroguzman, M.E.; Romouribe, A.; Zaratehernandez, B.M.; Cruzsilva, R. Viscoelastic properties of POSS-styrene nanocomposite blended with polystyrene. Rheol. Acta 2009, 48, 641–652. [Google Scholar] [CrossRef]
- Villani, V.; Lavallata, V. Rheology of lightly-cured polydimethylsiloxane liquid blends. Eur. Polym. J. 2019, 117, 434–441. [Google Scholar] [CrossRef]
- Teo, L.; Chen, C.; Kuo, J. Fourier Transform Infrared Spectroscopy Study on Effects of Temperature on Hydrogen Bonding in Amine-Containing Polyurethanes and Poly(urethane−urea)s. Macromolecules 1997, 30, 1793–1799. [Google Scholar] [CrossRef]
- Coleman, M.M.; Skrovanek, D.J.; Hu, J.; Painter, P.C. Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules 1988, 21, 59–65. [Google Scholar] [CrossRef]
- Yılgör, E.; Yılgör, I.; Yurtsever, E. Hydrogen bonding and polyurethane morphology. I. Quantum mechanical calculations of hydrogen bond energies and vibrational spectroscopy of model compounds. Polymer 2002, 43, 6551–6559. [Google Scholar] [CrossRef]
- Srichatrapimuk, V.W.; Cooper, S.L. Infrared thermal analysis of polyurethane block polymers. J. Macromol. Sci. Part B Phys. 1978, 15, 267–311. [Google Scholar] [CrossRef]










| Sample Code | Commercial Name | Mn | Mw | PDI | Softening Point (°C) |
|---|---|---|---|---|---|
| A01 | 558 | 538 | 751 | 1.40 | 24.14 |
| A02 | PF-2 | 680 | 1222 | 1.80 | 38 |
| A03 | 8218 | 828 | 1607 | 1.94 | 57.19 |
| A04 | 8219 | 930 | 1872 | 2.01 | 57.79 |
| A05 | M01 | 1272 | 4003 | 3.15 | 67.08 |
| Sample Code | tanδmax | tanδ Peak Position (°C) | Temperate Range For tanδ > 0.3 | TA (Tanδ > 1) | ||
|---|---|---|---|---|---|---|
| T1 (°C) | T2 (°C) | ∆T (°C) | ||||
| PBMA | 1.481 | 65.08 | 27.45 | 109.13 | 81.8 | 40.4 |
| B01 | 2.352 | 56.96 | 35.12 | 110.49 | 75.7 | 71.64 |
| B02 | 2.282 | 79.14 | 53.6 | 126.77 | 73.7 | 68.52 |
| B03 | 2.118 | 83.07 | 54.51 | 131.35 | 76.1 | 63.28 |
| B04 | 2.023 | 84.38 | 55.93 | 138.62 | 82.9 | 60.49 |
| B05 | 1.622 | 86.22 | 56.62 | 144.21 | 87.9 | 46.46 |
| Sample Code | Tg-tan δ (°C) | Sample Code | Tg-dsc (°C) | ∆T′ (°C) |
|---|---|---|---|---|
| B02 | 79.14 | A02 | 38 | 41.14 |
| B03 | 83.07 | A03 | 57.19 | 25.88 |
| B04 | 84.38 | A04 | 57.79 | 26.59 |
| B05 | 86.22 | A05 | 67.08 | 19.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, S.; Wan, S.; Niu, B.; He, X.; Zhang, R. Effect of Phenolic Resin Oligomer Motion Ability on Energy Dissipation of Poly (Butyl Methacrylate)/Phenolic Resins Composites. Polymers 2020, 12, 490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020490
Huang X, Chen S, Wan S, Niu B, He X, Zhang R. Effect of Phenolic Resin Oligomer Motion Ability on Energy Dissipation of Poly (Butyl Methacrylate)/Phenolic Resins Composites. Polymers. 2020; 12(2):490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020490
Chicago/Turabian StyleHuang, Xing, Songbo Chen, Songhan Wan, Ben Niu, Xianru He, and Rui Zhang. 2020. "Effect of Phenolic Resin Oligomer Motion Ability on Energy Dissipation of Poly (Butyl Methacrylate)/Phenolic Resins Composites" Polymers 12, no. 2: 490. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020490