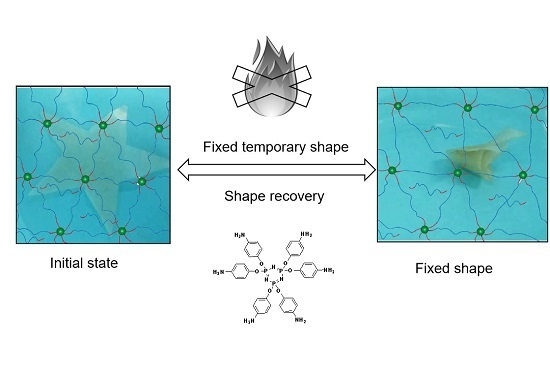
Star-Shaped Crosslinker for Multifunctional Shape Memory Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hexa(acetamidophenyl) Cyclotriphosphazene (CPAC)
2.3. Synthesis of Amine-Terminated Cyclophosphazene (ACP)
2.4. Preparation of Polyurethane Films with and without ACP
2.5. Methods
3. Results and Discussions
3.1. Structural Characterization
3.2. Thermal Behavior Analysis
3.3. Mechanical Performance Tests
3.3.1. Tensile Test
3.3.2. Shape Memory Cycles
3.4. Flame Retardant Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tong, K.; Amine Lehtihet, E.; Joshi, S. Parametric error modeling and software error compensation for rapid prototyping. Rapid Prototyp. J. 2003, 9, 301–313. [Google Scholar] [CrossRef]
- Felton, S.; Tolley, M.; Demaine, E.; Rus, D.; Wood, R. Applied origami. A method for building self-folding machines. Science 2014, 345, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, J.C.; Lewis, C.L.; Jiang, J.; Anthamatten, M. Photoinscription of Chain Anisotropy into Polymer Networks. Macromolecules 2016, 49, 9100–9107. [Google Scholar] [CrossRef]
- Zare, M.; Prabhakaran, M.P.; Parvin, N.; Ramakrishna, S. Thermally-induced two-way shape memory polymers: Mechanisms, structures, and applications. Chem. Eng. J. 2019, 374, 706–720. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49, 79–120. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.; Fang, Z.; Zou, W.; Zhao, Q.; Xie, T. Thermoset shape-memory polyurethane with intrinsic plasticity enabled by transcarbamoylation. Angew. Chem. Int. Ed. 2016, 55, 11421–11425. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Takahashi, T.; Hayashi, N.; Hayashi, S. Structure and properties of shape-memory polyurethane block copolymers. J. Appl. Polym. Sci. 1996, 60, 1061–1069. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Chen, S.; Ji, F. Theoretical study of hydrogen bonding interactions on MDI-based polyurethane. J. Mol. Model. 2010, 16, 1391–1399. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.; Xu, B.; Purnawali, H.; King, P.J.; Chalker, P.R.; Fu, Y.; Huang, W.; Miraftab, M. Synthesis and Characterization of Polyurethane-Based Shape-Memory Polymers for Tailored Tg around Body Temperature for Medical Applications. Macromol. Chem. Phys. 2011, 212, 592–602. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Li, X.; Han, J.; Ji, F. Architectural evolution of phase domains in shape memory polyurethanes by dissipative particle dynamics simulations. Polym. Chem. 2017, 8, 260–271. [Google Scholar] [CrossRef]
- Xie, T. Recent advances in polymer shape memory. Polymer 2011, 52, 4985–5000. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, I.A. Challenges of shape memory polymers: A review of the progress toward overcoming SMP’s limitations. Polym. Eng. Sci. 2008, 48, 2075–2089. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, P. Structure and Mechanical Properties of Shape Memory Polyurethane Based on Hyperbranched Polyesters. Polym. Bull. 2006, 57, 889–899. [Google Scholar] [CrossRef]
- Du, E.; Leng, S. Shape-Memory Polymers and Multifunctional Composites; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–386. [Google Scholar]
- Ding, Z.; Yuan, L.; Huang, T.; Liang, G.; Gu, A. High-Temperature Triple-Shape Memory Polymer with Full Recovery through Cross-Linking All-Aromatic Liquid Crystalline Poly(ester imide) under Reduced Molding Temperature. Ind. Eng. Chem. Res. 2019, 58, 8734–8742. [Google Scholar] [CrossRef]
- Çelebi, E.B.; Hacıvelioğlu, F. Preparation of sulfonic acid functional proton conducting phosphazenes by covalent protection. Polym. Chem. 2017, 8, 3022–3033. [Google Scholar] [CrossRef]
- Modesti, M.; Zanella, L.; Lorenzetti, A.; Bertani, R.; Gleria, M. Thermally stable hybrid foams based on cyclophosphazenes and polyurethanes. Polym. Degrad. Stabil. 2005, 87, 287–292. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Nguyen, M.M.; Al-Abdul-Wahid, M.S.; Easson, M.W.; Chang, S.; Lorigan, G.A.; Brian, D.C. The thermal degradation pathway studies of a phosphazene derivative on cotton fabric. Polym. Degrad. Stabil. 2015, 120, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Tang, X.; Huang, X.; Zheng, S. Synthesis, characterization and thermal properties of hexaarmed star-shaped poly (ε-caprolactone)-b-poly (d, l-lactide-co-glycolide) initiated with hydroxyl-terminated cyclotriphosphazene. Polymer 2005, 46, 1701–1707. [Google Scholar] [CrossRef]
- Du, G.; Mao, A.; Yu, J.; Hou, J.; Zhao, N.; Han, J.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Nacre-mimetic composite with intrinsic self-healing and shape-programming capability. Nat. Commun. 2019, 10, 800–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.J.; Huang, Z.C.; Li, B.; Xiao, W.X.; Zheng, E.; Yang, K.K.; Wang, Y.Z. A robust self-healing polyurethane elastomer: From H-bonds and stacking interactions to well-defined microphase morphology. Sci. China Mater. 2019, 62, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Fortman, D.J.; Brutman, J.P.; Hillmyer, M.A.; Dichtel, W.R. Structural effects on the reprocessability and stress relaxation of crosslinked polyhydroxyurethanes. J. Appl. Polym. Sci. 2017, 134, 44984–44994. [Google Scholar] [CrossRef] [Green Version]
- Rein, G.; Lautenberger, C.; Fernandez-Pello, A.C.; Torero, J.L.; Urban, D.L. Application of genetic algorithms and thermogravimetry to determine the kinetics of polyurethane foam in smoldering combustion. Combust. Flame 2006, 146, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Blasi, C.D.; Casu, A.; Morone, V.; Costa, C. Reaction kinetics and morphological changes of a rigid polyurethane foam during combustion. Thermochim. Acta 2003, 399, 127–137. [Google Scholar] [CrossRef]
- Gouri, M.E.; El Bachiri, A.; Hegazi, S.E.; Rafik, M.; El Harfi, A. Thermal degradation of a reactive flame retardant based on cyclotriphosphazene and its blend with DGEBA epoxy resin. Polym. Degrad. Stabil. 2009, 94, 2101–2106. [Google Scholar] [CrossRef]
- Marcano, A.; Fatyeyeva, K.; Koun, M.; Dubuis, P.; Grimme, M.; Marais, S. Recent developments in the field of barrier and permeability properties of segmented polyurethane elastomers. Rev. Chem. Eng. 2019, 35, 445–474. [Google Scholar] [CrossRef]
- Patel, R.H.; Patel, K.S. Synthesis and characterization of flame retardant hyperbranched polyurethanes for nano-composite and nano-coating applications. Prog. Org. Coat. 2015, 88, 283–292. [Google Scholar] [CrossRef]
- Hagen, R.; Salman, L.; Stenberg, B. Effects of the type of crosslink on viscoelastic properties of natural rubber. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1997–2006. [Google Scholar] [CrossRef]
- Huang, X.Y.; Cornelis, F.; Peng, X.P.; Xie, J.L.; Qi, J.Q.; Jiang, Y.Z.; Xiao, H.; Nie, S.X. Thermal Stability Analysis of Polyurethane Foams Made from Microwave Liquefaction Bio-Polyols with and Without Solid Residue. BioResources 2018, 13, 3346–3361. [Google Scholar] [CrossRef]
- Nelson, B.A.; King, W.P.; Gall, K. Shape recovery of nanoscale imprints in a thermoset ‘shape memory’ polymer. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, R.; Li, X.; Cheng, B.; Zhang, Y.; Yang, X.; Zhao, X.; Zhao, Y.; Huang, W. 4D printing of shape memory polyurethane via stereolithography. Eur. Polym. J. 2018, 101, 120–126. [Google Scholar] [CrossRef]
- Mya, K.Y.; Gose, H.B.; Pretsch, T.; Bothe, M.; He, C. Star-shaped POSS-polycaprolactone polyurethanes and their shape memory performance. J. Mater. Chem. 2011, 21, 4827–4836. [Google Scholar] [CrossRef]
- Li, M.; Guan, Q.; Dingemans, T.J. High-Temperature Shape Memory Behavior of Semicrystalline Polyamide Thermosets. ACS Appl. Mater. Interfaces 2018, 10, 19106–19115. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, F.; Hou, S.; Qi, H.J.; Yu, K. A viscoelastic model for hydrothermally activated malleable covalent network polymer and its application in shape memory analysis. J. Mech. Phys. Solids 2019, 127, 239–265. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, W.J.; Wang, J.S.; Li, F.; Liu, Y.Q. Synthesis of a novel bridged-cyclotriphosphazene flame retardant and its application in epoxy resin. Polym. Degrad. Stabil. 2016, 133, 162–173. [Google Scholar] [CrossRef]
- Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Yang, Y.; Wang, Y.; Wang, Z. A Novel Application of Phosphorene as a Flame Retardant. Polymers 2018, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Krishnadevi, K.; Selvaraj, V. Development of halogen-free flame retardant phosphazene and rice husk ash incorporated benzoxazine blended epoxy composites for microelectronic applications. New J. Chem. 2015, 39, 6555–6567. [Google Scholar] [CrossRef]






| Polyurethane | PPG-400 (mmol) | HDI (mmol) | ACP (mmol) | ACP (%) | Feeding Ratio | Stoichiometric Ratio |
|---|---|---|---|---|---|---|
| PU | 8.3 | 16.5 | 0 (2.2 *) | 0 | 1:2:0 | 1:2:0 |
| FSPU-5 | 8.3 | 16.5 | 0.41 | 5 | 1:2:0.05 | 1:2:0.167 |
| FSPU-10 | 8.3 | 16.5 | 0.83 | 10 | 1:2:0.10 | 1:2:0.167 |
| FSPU-15 | 8.3 | 16.5 | 1.24 | 15 | 1:2:0.15 | 1:2:0.167 |
| FSPU-20 | 8.3 | 16.5 | 1.63 | 20 | 1:2:0.20 | 1:2:0.167 |
| FSPU-30 | 8.3 | 16.5 | 2.45 | 30 | 1:2:0.30 | 1:2:0.167 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Toughness (MJ/m3) |
|---|---|---|---|
| PU | 2.53 ± 0.43 | 120.34 ± 12.8 | 239.58 ± 39.55 |
| FSPU-5 | 2.16 ± 0.03 | 161.51 ± 9.29 | 262.28 ± 57.47 |
| FSPU-10 | 2.03 ± 0.07 | 102.34 ± 10.7 | 154.43 ± 30.04 |
| Sample | Peak HR (W/g) | Total HR (KJ/g) | Tp (°C) |
|---|---|---|---|
| PU | 393.4 | 35.9 | 368.9 |
| FSPU-5 | 408.6 | 26.2 | 369.0 |
| FSPU-10 | 361.8 | 23.8 | 371.2 |
| FSPU-15 | 212.0 | 23.1 | 371.1 |
| FSPU-20 | 193.9 | 21.5 | 361.7 |
| FSPU-30 | 183.2 | 21.4 | 330.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Chi, H.; Li, Z.; Li, T.; Wang, F. Star-Shaped Crosslinker for Multifunctional Shape Memory Polyurethane. Polymers 2020, 12, 740. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040740
Song X, Chi H, Li Z, Li T, Wang F. Star-Shaped Crosslinker for Multifunctional Shape Memory Polyurethane. Polymers. 2020; 12(4):740. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040740
Chicago/Turabian StyleSong, Xiuhuan, Hong Chi, Zibiao Li, Tianduo Li, and FuKe Wang. 2020. "Star-Shaped Crosslinker for Multifunctional Shape Memory Polyurethane" Polymers 12, no. 4: 740. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040740