A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine
Abstract
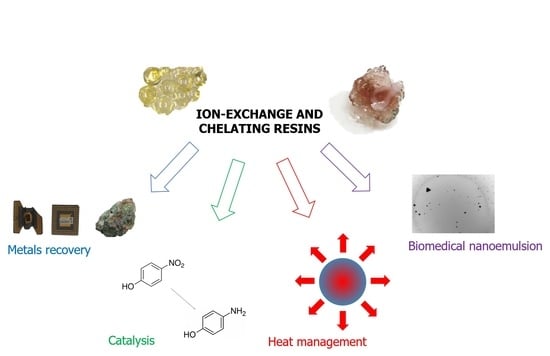
:1. Introduction
2. Synthetic Protocols for Hydrometallurgy
3. Nanocomposite Catalysts
3.1. In Situ Synthesis Approach
3.2. Cold Atmospheric Pressure Plasmas Ex Situ Approach
4. Summary
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115, 30–51. [Google Scholar] [CrossRef]
- Liang, P.; Yuana, L.; Yangb, X.; Zhouc, S.; Huang, X. Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionization cells for domestic wastewater desalination. Water Res. 2013, 47, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.D. Process for Preparing Chloromethylated Polystyrene-Divinylbenzene Copolymer. U.S. Patent 3872067, 18 March 1975. [Google Scholar]
- Parajuli, D.; Kunathai, K.; Adhikari, C.R.; Inoue, K.; Ohto, K.; Kawakita, H.; Funaoka, M.; Hirota, K. Total recovery of gold, palladium, and platinum using lignophenol derivative. Miner. Eng. 2009, 22, 1173–1178. [Google Scholar] [CrossRef]
- MarketsandMarkets™ Research Private Ltd. Available online: https://www.marketsandmarkets.com/pressreleases/ion-exchange-resins.Asp (accessed on 9 September 2019).
- Alexandratos, S.D. Ion-exchange resins: A retrospective from industrial and engineering chemistry research. Ind. Eng. Chem. Res. 2009, 48, 388–398. [Google Scholar] [CrossRef]
- Silva, R.A.; Hawboldt, K.; Zhang, Y. Application of resins with functional groups in the separation of metal ions/species–A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 395–413. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.-L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extr. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Hubicki, Z.; Wawrzkiewicz, M.; Wołowicz, A. Application of ion exchange methods in recovery of pd (ii) ions–A review. Chem. Anal. 2008, 53, 759–784. [Google Scholar]
- Cyganowski, P. Synthesis of adsorbents with anion exchange and chelating properties for separation and recovery of precious metals–A review. Solvent Extr. Ion Exch. 2020, 38, 143–165. [Google Scholar] [CrossRef]
- Concha-Barrientos, M.; Nelson, D.I.; Driscoll, T.; Steenland, N.K.; Punnett, L.; Fingerhut, M.A.; Prüss-Üstün, A.; Leigh, J.; Tak, S.; Corvalan, C. Selected Occupational Risk Factors in Comparative Quantification of Health Risk; World Health Organisation: Geneva, Switzerland, 2004; Volume 2. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Sibrell, P.; Atkinson, G.B.; Walters, L.A. Cyanide leaching chemistry of platinum-group metals. In Report of Investigations 9507; US Department of the Interior: Washington, WA, USA, 1994. [Google Scholar]
- Anthony, E.Y.; Williams, P.A. Thiosulfate complexing of platinum-group elements-implications for supergene geochemistry. Environ. Geochem. Sulfide Oxid. 1994, 550, 551–560. [Google Scholar]
- Mpinga, C.; Eksteen, J.; Aldrich, C.; Dyer, L. Direct leach approaches to platinum group metal (pgm) ores and concentrates: A review. Miner. Eng. 2015, 78, 93–113. [Google Scholar] [CrossRef]
- Dorfner, K. Ion Exchangers; Walter De Gruyter Inc.: Berlin, Germany, 1991. [Google Scholar]
- Subramonian, S. Anion-exchange resins from vinylbenzyl chloride-control of hydrolyzis during polymerization. React. Funct. Polym. 1996, 29, 129–133. [Google Scholar] [CrossRef]
- Ezzeldin, H.A.; Apblett, A.; Foutch, G.L. Synthesis and properties of anion exchangers derived from chloromethyl styrene codivinylbenzene and their use in water treatment. Int. J. Polym. Sci. 2010, 1–9. [Google Scholar] [CrossRef]
- Tassi, M.; Bartollini, E.; Adriaensens, P.; Bianchi, L.; Barkakaty, B.; Carleer, R.; Chen, J.; Hensley, D.K.; Marrocchi, A.; Vaccaro, L. Synthesis, characterization and catalytic activity of novel large network polystyrene-immobilized organic bases. RSC Adv. 2015, 5, 107200–107208. [Google Scholar] [CrossRef]
- Elkady, M.F.; Mahmoud, M.M.; Abd-El-Rahman, H.M. Kinetic approach for cadmium sorption using microwave synthesized nano-hydroxyapatite. J. Non Cryst. Solids 2011, 357, 1118–1129. [Google Scholar] [CrossRef]
- Loupy, A. Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Mondal, B.C.; Das, A.K. Microwave-assisted synthesis of a new chelating resin containing 2-aminothiophenyl s-acetic acid and its application to the determination of lead. React. Funct. Polym. 2001, 53, 45–52. [Google Scholar] [CrossRef]
- Clark, D.E.; Folz, D.C.; Folgar, C.E.; Mahmoud, M.M. Microwave Solutions for Ceramic Engineers; American Ceramic Society: Westerville, OH, USA, 2005. [Google Scholar]
- Bogdal, D.; Penczek, P.; Pielichowski, J.; Prociak, A. Microwave assisted synthesis, crosslinking, and processing of polymeric materials. In Liquid Chromatography/Ftir Microspectroscopy/Microwave Assisted Synthesis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 194–263. [Google Scholar]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D. Piperazine functionalized resins for au(iii), pt(iv), and pd(ii) sorption. Sep. Sci. Technol. 2014, 49, 1689–1699. [Google Scholar] [CrossRef]
- Cyganowski, P.; Cierlik, A.; Leśniewicz, A.; Pohl, P.; Jermakowicz-Bartkowiak, D. Separation of re (vii) from mo (vi) by anion exchange resins synthesized using microwave heat. Hydrometallurgy 2019, 185, 12–22. [Google Scholar] [CrossRef]
- Jermakowicz-Bartkowiak, D.; Cyganowski, P. Effect of microwave radiation on the synthesis of ion exchange resins: A comparative study. Solvent Extr. Ion Exch. 2015, 33, 510–521. [Google Scholar] [CrossRef]
- Jermakowicz-Bartkowiak, D.; Cyganowski, P.; Kawałko, J. Microwave-assisted synthesis of anion-exchange resins for sorption of noble metals: How to boost sorption capacity using a proper reaction environment. Polym. Bull. 2017, 74, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D. Experimental review of microwave-assisted methods for synthesis of functional resins for sorption of rhenium(vii). Solvent Extr. Ion Exch. 2018, 36, 420–436. [Google Scholar] [CrossRef]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Sharipova, A.S.; Sadykanova, S.E.; Bochevskaya, Y.G.; Atanova, O.V. Sorption of rhenium and uranium by strong base anion exchange resin from solutions with different anion compositions. Hydrometallurgy 2013, 131, 127–132. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.-Z.; Wang, W.; Gao, Z.-G.; Cao, Y.-H. Recovery of rhenium from copper leach solutions using ion exchange with weak base resins. Hydrometallurgy 2017, 173, 50–56. [Google Scholar] [CrossRef]
- Jermakowicz-Bartkowiak, D.; Kolarz, B.N. Poly(4-vinylpyridine) resins towards perrhenate sorption and desorption. React. Funct. Polym. 2011, 71, 95–103. [Google Scholar] [CrossRef]
- Fathi, M.B.; Rezai, B.; Alamdari, E.K. Competitive adsorption characteristics of rhenium in single and binary (re-mo) systems using purolite a170. Int. J. Miner. Process. 2017, 169, 1–6. [Google Scholar] [CrossRef]
- Xiong, C.; Yao, C.; Wu, X. Adsorption of rhenium(vii) on 4-amino-1,2,4-triazole resin. Hydrometallurgy 2008, 90, 221–226. [Google Scholar] [CrossRef]
- Jia, M.; Cui, H.; Jin, W.; Zhu, L.; Liu, Y.; Chen, J. Adsorption and separation of rhenium(vii) using n-methylimidazolium functionalized strong basic anion exchange resin. J. Chem. Technol. Biotechnol. 2013, 88, 437–443. [Google Scholar] [CrossRef]
- European Association of Mining Industries, Metal Ores & Industrial Minerals. Available online: http://www.euromines.org/news/eu-commission-confirms-sufficient-legislation-place-gold (accessed on 17 January 2017).
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D. Synthesis and studies on core–shell type anion exchange resins based on a hybrid polymeric support. J. Appl. Polym. Sci. 2016, 133, 43841. [Google Scholar] [CrossRef]
- Cyganowski, P.; Garbera, K.; Leśniewicz, A.; Wolska, J.; Pohl, P.; Jermakowicz-Bartkowiak, D. The recovery of gold from the aqua regia leachate of electronic parts using a core–Shell type anion exchange resin. J. Saudi Chem. Soc. 2017, 21, 741–750. [Google Scholar] [CrossRef]
- Das Graças Santos, N.T.; Moraes, L.F.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of gold through adsorption onto sericin and alginate particles chemically crosslinked by proanthocyanidins. J. Clean. Prod. 2020, 253, 119925. [Google Scholar] [CrossRef]
- Zhang, H.; Jeffery, C.A.; Jeffrey, M.I. Ion exchange recovery of gold from iodine–iodide solutions. Hydrometallurgy 2012, 125, 69–75. [Google Scholar] [CrossRef]
- Panda, R.; Dinkar, O.S.; Jha, M.K.; Pathak, D.D. Recycling of gold from waste electronic components of devices. Korean J. Chem. Eng. 2020, 37, 111–119. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Recovery of gold (iii) from the stripping solution containing palladium (ii) by ion exchange and synthesis of gold particles. J. Ind. Eng. Chem. 2019, 69, 255–262. [Google Scholar] [CrossRef]
- Cyganowski, P.; Lesniewicz, A.; Dzimitrowicz, A.; Wolska, J.; Pohl, P.; Jermakowicz-Bartkowiak, D. Molecular reactors for synthesis of polymeric nanocomposites with noble metal nanoparticles for catalytic decomposition of 4-nitrophenol. J. Colloid Interface Sci. 2019, 541, 226–233. [Google Scholar] [CrossRef]
- Cyganowski, P.; Leśniewicz, A.; Polowczyk, I.; Chęcmanowski, J.; Koźlecki, T.; Pohl, P.; Jermakowicz-Bartkowiak, D. Surface-activated anion exchange resins for synthesis and immobilization of gold and palladium nano- and microstructures. React. Funct. Polym. 2018, 124, 90–103. [Google Scholar] [CrossRef]
- Newman, J.; Blanchard, G. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Leśniewicz, A.; Pohl, P.; Dzimitrowicz, A. Highly efficient and convenient nanocomposite catalysts produced using in-situ approach for decomposition of 4-nitrophenol. Colloids Surf. A 2020, 590, 124452. [Google Scholar] [CrossRef]
- Jana, S.; Ghosh, S.K.; Nath, S.; Pande, S.; Praharaj, S.; Panigrahi, S.; Basu, S.; Endo, T.; Pal, T. Synthesis of silver nanoshell-coated cationic polystyrene beads: A solid phase catalyst for the reduction of 4-nitrophenol. Appl. Catal. A Gen. 2006, 313, 41–48. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Kebe, S.I.; Mahouche-Chergui, S.; Guerrouache, M.; Carbonnier, B.; Jaziri, M.; Chehimi, M.M. Aryl diazonium-modified olive waste: A low cost support for the immobilization of nanocatalysts. Colloid Surf. A 2017, 529, 541–549. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over au nanoparticles deposited on pmma. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Ma, M.; Yang, Y.; Li, W.; Feng, R.; Li, Z.; Lyu, P.; Ma, Y. Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J. Mater. Sci. 2019, 54, 323–334. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, X.; Wu, Y.; Yan, L.; Du, J.; Dai, J.; Xiong, Q.; Guo, Y.; Xiao, D. Surfactant-free gold nanoparticles: Rapid and green synthesis and their greatly improved catalytic activities for 4-nitrophenol reduction. Inorg. Chem. Front. 2017, 4, 1268–1272. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, F.; Wang, W.; Qiu, X.; Qiao, X.; Chen, J. Facile, template-free synthesis of silver nanodendrites with high catalytic activity for the reduction of p-nitrophenol. J. Hazard. Mater. 2012, 217, 36–42. [Google Scholar] [CrossRef]
- Acosta, B.; Evangelista, V.; Miridonov, S.; Fuentes, S.; Simakov, A. The decoration of gold core in au@ zro 2 nanoreactors with trace amounts of pd for the effective reduction of 4-nitrophenol to 4-aminophenol. Catal. Lett. 2019, 149, 1621–1632. [Google Scholar] [CrossRef]
- Starek, A.; Pawłat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Li, J.; Li, Y. A review of plasma–liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef] [Green Version]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Reuter, S.; Von Woedtke, T.; Weltmann, K.-D. The kinpen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Bratescu, M.A.; Cho, S.-P.; Takai, O.; Saito, N. Size-controlled gold nanoparticles synthesized in solution plasma. J. Phys. Chem. C 2011, 115, 24569–24576. [Google Scholar] [CrossRef]
- Caroline De, V.; Joffrey, B.; Megan, W.; Jean, D.; Stéphane, G.; Michael, J.G.; Sankaran, R.M.; François, R. A comparative study of the reduction of silver and gold salts in water by a cathodic microplasma electrode. J. Phys. D Appl. Phys. 2017, 50, 105206. [Google Scholar]
- Huang, X.; Li, Y.; Zhong, X. Effect of experimental conditions on size control of au nanoparticles synthesized by atmospheric microplasma electrochemistry. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Kim, G.S.; Lee, S.Y. Effects of pvp and kcl concentrations on the synthesis of gold nanoparticles using a solution plasma processing. Mater. Lett. 2008, 62, 4354–4356. [Google Scholar] [CrossRef]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma–liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process. Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Richmonds, C.; Sankaran, R.M. Plasma-liquid electrochemistry: Rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl. Phys. Lett. 2008, 93, 131501. [Google Scholar] [CrossRef]
- Naoki, S.; Satoshi, U.; Fumiyoshi, T. Synthesis of metal nanoparticles by dual plasma electrolysis using atmospheric dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 046202. [Google Scholar]
- Fumiyoshi, T.; Yudai, S.; Naoki, S.; Satoshi, U. Chemical reactions in liquid induced by atmospheric-pressure dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 126201. [Google Scholar]
- Ruixue, W.; Shasha, Z.; Dong, W.; Jue, Z.; Weidong, Z.; Becker, K.H.; Jing, F. Microplasma-assisted synthesis of colloidal gold nanoparticles and their use in the detection of cardiac troponin i (ctn-i). Plasma Process. Polym. 2015, 12, 380–391. [Google Scholar]
- Thong, Y.L.; Chin, O.H.; Ong, B.H.; Huang, N.M. Synthesis of silver nanoparticles prepared in aqueous solutions using helium dc microplasma jet. Jpn. J. Appl. Phys. 2015, 55, 01AE19. [Google Scholar] [CrossRef]
- Dong, P.; Yang, F.; Cheng, X.; Huang, Z.; Nie, X.; Xiao, Y.; Zhang, X. Plasmon enhanced photocatalytic and antimicrobial activities of ag-tio2 nanocomposites under visible light irradiation prepared by dbd cold plasma treatment. Mater. Sci. Eng. C 2019, 96, 197–204. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Lesniewicz, T.; Greda, K.; Jamroz, P.; Nyk, M.; Pohl, P. Production of gold nanoparticles using atmospheric pressure glow microdischarge generated in contact with a flowing liquid cathode-A design of experiments study. RSC Adv. 2015, 5, 90534–90541. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; Greda, K.; Nowak, P.; Nyk, M.; Pohl, P. The influence of stabilizers on the production of gold nanoparticles by direct current atmospheric pressure glow microdischarge generated in contact with liquid flowing cathode. J. Nanopart. Res. 2015, 17, 185. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Greda, K.; Lesniewicz, T.; Jamroz, P.; Nyk, M.; Pohl, P. Size-controlled synthesis of gold nanoparticles by a novel atmospheric pressure glow discharge system with a metallic pin electrode and a flowing liquid electrode. RSC Adv. 2016, 6, 80773–80783. [Google Scholar] [CrossRef] [Green Version]
- Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P.; Pohl, P.; Dzimitrowicz, A. Hydrogel-based nanocomposite catalyst containing uncoated gold nanoparticles synthesized using cold atmospheric pressure plasma for the catalytic decomposition of 4-nitrophenol. Colloids Surf. A 2019, 582, 123886. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamroz, P.; Pogoda, D.; Nyk, M.; Pohl, P. Direct current atmospheric pressure glow discharge generated between a pin-type solid cathode and a flowing liquid anode as a new tool for silver nanoparticles production. Plasma Process. Polym. 2017, 14, 1600251. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; diCenzo, G.; Jamroz, P.; Macioszczyk, J.; Klimczak, A.; Pohl, P. Pulse-modulated radio-frequency alternating-current-driven atmospheric-pressure glow discharge for continuous-flow synthesis of silver nanoparticles and evaluation of their cytotoxicity toward human melanoma cells. Nanomaterials 2018, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Motyka-Pomagruk, A.; Cyganowski, P.; Babinska, W.; Terefinko, D.; Jamroz, P.; Lojkowska, E.; Pohl, P.; Sledz, W. Antibacterial activity of fructose-stabilized silver nanoparticles produced by direct current atmospheric pressure glow discharge towards quarantine pests. Nanomaterials 2018, 8, 751. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Krychowiak-Masnicka, M.; Pohl, P.; Krolicka, A.; Cyganowski, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P. Production of antimicrobial silver nanoparticles modified by alkanethiol self-assembled monolayers by direct current atmospheric pressure glow discharge generated in contact with a flowing liquid anode. Plasma Process. Polym. 2019, 16, 1900033. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Motyka, A.; Jamroz, P.; Lojkowska, E.; Babinska, W.; Terefinko, D.; Pohl, P.; Sledz, W. Application of silver nanostructures synthesized by cold atmospheric pressure plasma for inactivation of bacterial phytopathogens from the genera Dickeya and Pectobacterium. Materials 2018, 11, 331. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Jamroz, P.; Nyk, M.; Pohl, P. Application of direct current atmospheric pressure glow microdischarge generated in contact with a flowing liquid solution for synthesis of Au-Ag core-shell nanoparticles. Materials 2016, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Cyganowski, P.; Pohl, P.; Jermakowicz-Bartkowiak, D.; Terefinko, D.; Jamroz, P. Atmospheric pressure plasma-mediated synthesis of platinum nanoparticles stabilized by poly(vinylpyrrolidone) with application in heat management systems for internal combustion chambers. Nanomaterials 2018, 8, 619. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; Pohl, P.; Jermakowicz-Bartkowiak, D.; Jamroz, P.; Malik-Gajewska, M.; Klimczak, A.; Cyganowski, P. Application of oil-in-water nanoemulsion carrying size-defined gold nanoparticles synthesized by non-thermal plasma for the human breast cancer cell lines migration and apoptosis. Plasma Chem. Plasma Process. 2020, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wei-Hung, C.; Carolyn, R.; Sankaran, R.M. Continuous-flow, atmospheric-pressure microplasmas: A versatile source for metal nanoparticle synthesis in the gas or liquid phase. Plasma Sources Sci. Technol. 2010, 19, 034011. [Google Scholar]
- Patel, J.; Němcová, L.; Maguire, P.; Graham, W.G.; Mariotti, D. Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef] [Green Version]
- Cyganowski, P.; Dzimitrowicz, A.; Jamroz, P.; Jermakowicz-Bartkowiak, D.; Pohl, P. Polymerization-driven immobilization of dc-apgd synthesized gold nanoparticles into a quaternary ammonium-based hydrogel resulting in a polymeric nanocomposite with heat-transfer applications. Polymers 2018, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Bhanvase, B.A.; Kamath, S.D.; Patil, U.P.; Patil, H.A.; Pandit, A.B.; Sonawane, S.H. Intensification of heat transfer using pani nanoparticles and pani-cuo nanocomposite based nanofluids. Chem. Eng. Process. Process Intensif. 2016, 104, 172–180. [Google Scholar] [CrossRef]
- Lo, C.-W.; Zhu, D.; Jiang, H. An infrared-light responsive graphene-oxide incorporated poly(n-isopropylacrylamide) hydrogel nanocomposite. Soft Matter 2011, 7, 5604–5609. [Google Scholar] [CrossRef]
- Li, T.; Lee, J.-H.; Wang, R.; Kang, Y.T. Enhancement of heat transfer for thermal energy storage application using stearic acid nanocomposite with multi-walled carbon nanotubes. Energy 2013, 55, 752–761. [Google Scholar] [CrossRef]
- Ding, Q.; Kang, Z.; Cao, L.; Lin, M.; Lin, H.; Yang, D.-P. Conversion of waste eggshell into difunctional au/caco3 nanocomposite for 4-nitrophenol electrochemical detection and catalytic reduction. Appl. Surf. Sci. 2020, 510, 145526. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, K.; Guo, J.; Li, J.; Peng, X.; Hong, B.; Wang, X.; Ge, H. Facile fabrication of au/fe3o4 nanocomposites as excellent nanocatalyst for ultrafast recyclable reduction of 4-nitropheol. Chem. Eng. J. 2020, 381, 122596. [Google Scholar] [CrossRef]
- Balakumar, V.; Kim, H.; Ryu, J.W.; Manivannan, R.; Son, Y.-A. Uniform assembly of gold nanoparticles on s-doped g-c3n4 nanocomposite for effective conversion of 4-nitrophenol by catalytic reduction. J. Mater. Sci. Technol. 2020, 40, 176–184. [Google Scholar] [CrossRef]
- Huang, R.-F.S.; Wei, Y.-J.; Inbaraj, B.S.; Chen, B.-H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823. [Google Scholar]
- Fornaguera, C.; Feiner-Gracia, N.; Dols-Perez, A.; García-Celma, M.J.; Solans, C. Versatile methodology to encapsulate gold nanoparticles in plga nanoparticles obtained by nano-emulsion templating. Pharm. Res. 2017, 34, 1093–1103. [Google Scholar] [CrossRef]
- Guler, E.; Barlas, F.B.; Yavuz, M.; Demir, B.; Gumus, Z.P.; Baspinar, Y.; Coskunol, H.; Timur, S. Bio-active nanoemulsions enriched with gold nanoparticle, marigold extracts and lipoic acid: In vitro investigations. Colloids Surf. B Biointerfaces 2014, 121, 299–306. [Google Scholar] [CrossRef]










| Copolymer. | Method of Synthesis | Synthesis Time | Conversion of Monomers | Hydrolysis of VBC | Cl 1 |
|---|---|---|---|---|---|
| VBC-co-DVB | Conventional | 25 h | 70% | + | 4.63 ± 0.04 |
| MV-assisted | 4 h | 90% | − | 5.24 ± 0.04 |
| System | Polymeric Base | Active Sites | Re(VII) Uptake | Comments | Ref. |
|---|---|---|---|---|---|
| Re + U | Ambersep A920U | Strong base | 37 mg g−1 | Possible Re and U separation | [31] |
| Re + As + Cu + Fe + Si | ZS70 | Weak base | 3.15 mg g−1 | Effective Re separation | [32] |
| Re | Quaternized poly(4-vinyl)pyridine-co-divinylbenzene | 61 mg g−1 | Up to 170 mg g−1 Re(VII) uptake during column studies | [33] | |
| Reliex 436 | Strong base | 46 mg g−1 | |||
| Re + Mo | Purolite A170 | Weak base | 167 mg g−1 | Separation of Re and Mo almost impossible. | [34] |
| Re + Mo | S-co-DVB | 4-amino-1,2,4-triazol | 354 mg g−1 | 17-fold greater affinity toward Re(VII) when compared to Mo(VI) | [35] |
| Re + Mo | Merrifield resin | N-methylimidazole | 462 mg g−1 | Resin reveals affinity toward Mo(VI) | [36] |
| Re + Mo + V + Cu | VBC-co-DVB | 1-(3-aminopropyl)imidazole | 303 mg g−1 | Resins obtained applying microwave heat. Affinity toward Re(VII) 200-fold greater when compared to Mo(VI). No adsorption of V and Cu observed | [27] * |
| Au(III) uptake | |||||
| Au iodine–iodide | Purolute A500Plus (S-co-DVB) | Strong base | 17 g dm−3 | Possible 97% of Au recovery from a resin | [43] |
| Au | Sericin and alginate crosslinked particles | 137–648 mg g−1 | 78–84% recovery, using thiourea in HCl | [42] | |
| Au cyanide leachate of PCB | Amberlite IRA400Cl | Strong base | 11 mg g−1 | Up to 100% recovery of Au, using thiourea stripping agent | [44] |
| Au + Pb | AmberliteXAD7HP | 14 mg g−1 | Reduction-coupled recovery of Au from Pb-based stripping agent | [45] | |
| Au aqua regia leachate of WEEE | Amberlite XAD-4 (S-co-DVB) | 1-(2-aminoethyl)piperazine loaded on the core–shell structure | 20 mg g−1 | Resins selective toward trace-quantities of Au, resistant to aqua regia, 100% Au recovery | [41] * |
| Support | Nanoparticles | Increase of Heat Transfer Rate | Ref. |
|---|---|---|---|
| polyaniline | CuO | 12–38% | [88] |
| poly(N-isopropylacrylamide) | Graphene oxide | ~200% | [89] |
| stearic acid | Carbon nanotubes | 91% | [90] |
| - | Cu, Al2O3 nanofluid | 80% | [27,30] |
| Poly(vinyl pyrrolidone) | PtNPs | 800% | [81] * |
| (vinylbenzyl)trimethylammonium chloride-co-N,N-methylene bisacrylamide | AuNPs | 300% | [87] * |
| Support | Average Size of AuNPs (nm) | km (mg−1 min−1) | Ref. |
|---|---|---|---|
| Aryl diazonium-modified olive waste | n.a. | 0.02 | [51] |
| PMMA | 7.0 | 0.37 | [52] |
| Magnetite microspheres | 5.0–7.0 | 1.22 | [53] |
| Reductive carbon dots | 15.0 | 0.10 | [54] |
| ZrO2 | 17.0 ± 5.1 | 0.06 | [56] |
| Egg-shell | 6.2 ± 4.0 | 1.07 | [91] |
| Fe3O4 | 15.0 | 13.8 | [92] |
| Sulfur doped graphitic carbon nitride | 12.0 | 1.50 | [93] |
| Quaternary ammonium hydrogel | 6.2 ± 1.2 | 0.10 | [76] * |
| VBC/DVB with 1-amino-4-methylpiperazine ligands | 4.5 ± 2.1 | 1.46 | [49] * |
| Components of Reaction Mixture | Average Size of AuNPs (nm) | Applications | Ref. |
|---|---|---|---|
| AuNPs, Polysorbate 80, PLGA | 15 | Biomedical, not defined | [95] |
| AuNPs, Calendula officinalis, Tween 85, Nigella sativa oil, lecithin | 40 | Bioactive agent | [96] |
| AuNPs, Tween 80, Lycopene | 21.3 ± 3.7 | Colon cancer cells | [94] |
| AuNPs, Gelatin, Turmeric oil | 4.6 ± 1.0 | Breast cancer cells | [84] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyganowski, P.; Dzimitrowicz, A. A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine. Polymers 2020, 12, 784. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040784
Cyganowski P, Dzimitrowicz A. A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine. Polymers. 2020; 12(4):784. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040784
Chicago/Turabian StyleCyganowski, Piotr, and Anna Dzimitrowicz. 2020. "A Mini-Review on Anion Exchange and Chelating Polymers for Applications in Hydrometallurgy, Environmental Protection, and Biomedicine" Polymers 12, no. 4: 784. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040784