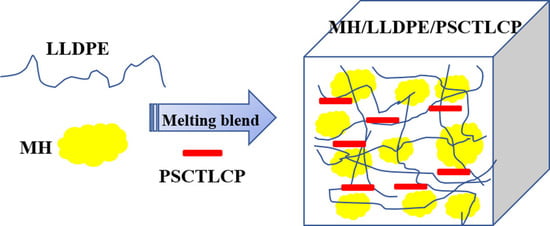
Design and Synthesis of Polysiloxane Based Side Chain Liquid Crystal Polymer for Improving the Processability and Toughness of Magnesium Hydrate/Linear Low-Density Polyethylene Composites
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of 4-allyloxybenzoic Acid (AOBA)
2.3. Synthesis of 4-allyloxybenzoyl Chloride
2.4. Synthesis of 4-hydroxyphenyl-4-ethylbenzoate (HPEB)
2.5. Synthesis of 4-ethylbenzoic Acid-4-allyloxybenzoic Acid Hydroquinone Diester (M)
2.6. Synthesis of PSCTLCP
2.7. Preparation of MH/LLDPE/PSCTLCP Composites
2.8. Characterization
2.8.1. Structural Characterization
2.8.2. Differential Scanning Calorimetry (DSC)
2.8.3. Polarized Light Optical Microscopy (POM)
2.8.4. Thermogravimetric (TG)
2.8.5. Torque Rheometer
2.8.6. High Pressure Capillary Rheometer
2.8.7. Scanning Electron Microscope (SEM)
2.8.8. Tensile Testing
3. Results and Discussion
3.1. Characterization of PSCTLCP
3.2. Processability of MH/LLDPE/PSCTLCP Composites
3.3. The Facture Morphology of MH/LLDPE/PSCTLCP Composites
3.4. Mechanical Properties of MH/LLDPE/PSCTLCP Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PSCTLCP | polysiloxane grafted by thermotropic liquid crystal polymer; |
| MH | magnesium hydroxide; |
| LLDPE | linear low-density polyethylene; |
| PDMS | polydimethylsiloxane; |
| TLCP | Thermotropic liquid crystal polymer; |
| PIHQ | 2-(a-phenylisopropyl) hydroquinone; |
| AOBA | 4-allyloxybenzoic acid; |
| HPEB | 4-hydroxyphenyl-4′-ethylbenzoate; |
| M | 4-ethylbenzoic acid-4-allyloxybenzoic acid hydroquinone diester; |
| DSC | Differential scanning calorimetry; |
| POM | Polarized light optical microscopy; |
| TG | Thermogravimetric; |
| SEM | Scanning electron microscope; |
| PMHS | poly(methylhydrogeno)siloxane |
| DMAP | 4-dimethylaminopyridine; |
| (Et)3N | triethylamine; |
| DMF | N, N-dimethylformamide; |
| THF | tetrahydrofuran. |
References
- Kartik, B.; Mithilgsh, Y.; Fang, C.C.; Kyong, Y.R. Graphene Nanoplatelet-Reinforced Poly(vinylidene fluoride)/High Density Polyethylene Blend-Based Nanocomposites with Enhanced Thermal and Electrical Properties. Nanomaterials 2019, 9, 361. [Google Scholar]
- Liu, C.; Chan, K.; Shen, J. Polyetheretherketone Hybrid Composites with Bioactive Nanohydroxyapatite and Multiwalled Carbon Nanotube Fillers. Polymers 2016, 8, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartik, B.; Yen, H.C.; Mithilesh, Y.; Fang, C.C. Enhanced thermal stability, toughness, and electrical conductivity of carbon nanotube-reinforced biodegradable poly(lactic acid)/poly(ethylene oxide) blend-based nanocomposites. Polymer 2020, 186, 122002. [Google Scholar]
- Shen, L.; Li, J.; Li, R. A new strategy to produce low-density polyethylene (LDPE)-based composites simultaneously with high flame retardancy and high mechanical properties. Appl. Surf. Sci. 2017, 437, 75–81. [Google Scholar] [CrossRef]
- Shen, L.; Shao, C.; Li, R. Preparation and characterization of ethylene-vinyl acetate copolymer (EVA)-magnesium hydroxide (MH)-hexaphenoxycyclotriphosphazene (HPCTP) composite flame-retardant materials. Polym. Bull. 2018, 76, 2399–2410. [Google Scholar] [CrossRef]
- Liang, J.Z.; Yang, J.; Tang, C.Y. Melt shear viscosity of PP/Al(OH)3/Mg(OH)2 flame retardant composites at high extrusion rates. J. Appl. Polym. Sci. 2011, 119, 1835–1841. [Google Scholar] [CrossRef]
- Yang, J.; Liang, J.Z.; Tang, C.Y. Studies on melt flow properties during capillary extrusion of PP/Al(OH)3/Mg(OH)2 flame retardant composites. Polym. Test. 2009, 28, 907–911. [Google Scholar] [CrossRef]
- Liang, J.Z.; Tang, C.Y.; Zhang, Y.J. Melt density and volume flow rate of polypropylene/Al(OH)3/Mg(OH)2 flame retardant composites. J. Appl. Polym. Sci. 2010, 118, 331–337. [Google Scholar] [CrossRef]
- Cardelli, A.; Ruggeri, G.; Calderisi, M. Effects of poly(dimethylsiloxane) and inorganic fillers in halogen free flame retardant poly(ethylene-co-vinyl acetate) compound: A chemometric approach. Polym. Degrad. Stabil. 2012, 97, 2536–2544. [Google Scholar] [CrossRef]
- Zhou, W.; Osby, J. Siloxane modification of polycarbonate for superior flow and impact toughness. Polymer 2010, 51, 1990–1999. [Google Scholar] [CrossRef]
- Zolper, T.J.; Seyam, A.; Li, Z. Friction and Wear Protection Performance of Synthetic Siloxane Lubricants. Tribol. Lett. 2013, 51, 365–376. [Google Scholar] [CrossRef]
- Kokuti, Z.; Gruijthuijsen, K.V.; Jenei, M. High-frequency rheology of a high viscosity silicone oil using diffusing wave spectroscopy. Appl. Rheol. 2014, 24, 63984. [Google Scholar]
- He, H.M.; Gao, L.; Yang, X.J. Studies on the superhydrophobic properties of polypropylene/polydimethylsiloxane/graphite fluoride composites. J. Fluorine Chem. 2013, 156, 158–163. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Guo, S. Structure and properties of polypropylene composites filled with magnesium hydroxide. J. Appl. Polym. Sci. 2006, 102, 4943–4951. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Basu, T.; Sahoo, N.G.; Li, L.; Chan, S.H. Current Advances in the Carbon Nanotube/Thermotropic Main-Chain Liquid Crystalline Polymer Nanocomposites and Their Blends. Polymers 2012, 4, 889–912. [Google Scholar] [CrossRef]
- Zeng, L.; Li, R.; Chen, P. Synthesis and characterization of thermotropic liquid crystalline polyarylate with ether ether ketone segments in the main chain. J. Appl. Polym. Sci. 2016, 133, 43800. [Google Scholar] [CrossRef]
- Ren, C.; Gao, P. Synthesis and characterization of thermotropic liquid crystalline copolyester/multi-walled carbon nanotubes composites via in situ polymerization. Polymer 2012, 53, 3958–3967. [Google Scholar] [CrossRef]
- Garcia, M.; Gonzalez, N.; Eguiazabal, J.I. Structure and mechanical properties of new hybrid composites based on polyamide 6,6 reinforced with both glass fibers and a semiaromatic liquid crystalline polyester. Polym. Compos. 2004, 25, 601–608. [Google Scholar] [CrossRef]
- Pisharath, S.; Wong, S.C. Processability of LCP-nylon-glass hybrid composites. Polym. Compos. 2003, 24, 109–118. [Google Scholar] [CrossRef]
- Wu, L.; Chen, P.; Chen, J. Noticeable viscosity reduction of polycarbonate melts caused jointly by nano-silica filling and TLCP fibrillation. Polym. Eng. Sci. 2007, 47, 757–764. [Google Scholar] [CrossRef]
- Kalkar, A.K.; Deshpande, V.D.; Kulkarni, M.J. Nonisothermal crystallization kinetics of poly (phenylene sulphide) in composites with a liquid crystalline polymer. J. Polym. Sci. Pol. Phys. 2010, 48, 1070–1100. [Google Scholar] [CrossRef]
- Yang, R.; Chen, L.; Zhang, W.Q. In situ reinforced and flame-retarded polycarbonate by a novel phosphorus-containing thermotropic liquid crystalline copolyester. Polymer 2011, 52, 4150–4157. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Wang, Q. Study on in situ reinforced composites of thermoplastic resins: A novel TLCP with low melting temperature. J. Thermoplast. Compos. 2016, 29, 37–47. [Google Scholar] [CrossRef]
- Antoun, S.; Lenz, R.W.; Jin, J.I. Liquid crystal polymers. IV. Thermotropic polyesters with flexible spacers in the main chain. J. Polym. Sci. Pol. Chem. 1981, 19, 1901–1920. [Google Scholar] [CrossRef]
- Song, J.Y. Synthesis and characterization of a series of wholly aromatic copolyesters containing 2-(α-phenylisopropyl) hydroquinone moiety. J. Polym. Sci. Pol. Chem. 1999, 37, 881–889. [Google Scholar] [CrossRef]
- Zhao, C.S.; Chen, L.; Wang, Y.Z. A phosphorus-containing thermotropic liquid crystalline copolyester with low mesophase temperature and high flame retardance. J. Polym. Sci. Pol. Chem. 2008, 46, 5752–5759. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Morphology and mechanical characteristics of compatibilized polyamide 6-liquid crystalline polymer composites. Polymer 1997, 38, 4609–4615. [Google Scholar] [CrossRef]
- Laurence, N.; Hakima, M.J. Richness of Side-Chain Liquid-Crystal Polymers: From Isotropic Phase towards the Identification of Neglected Solid-Like Properties in Liquids. Polymers 2012, 4, 1109–1124. [Google Scholar]
- Zhang, L.; Yao, W.; Gao, Y. Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure-Phase Transition Behavior Relationships. Polymers 2018, 10, 794. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.F.; Shen, Z.; Wan, X.H. Mesogen-jacketed liquid crystalline polymers. Chem. Soc. Rev. 2010, 39, 3072. [Google Scholar] [CrossRef]
- Apfel, M.A.; Finkelmann, H. Synthesis and properties of high-temperature mesomorphic polysiloxane (MEPSIL) solvents: Biphenyl- and terphenyl-based nematic systems. Anal. Chem. 1985, 57, 651–658. [Google Scholar] [CrossRef]
- Jin, Y.; Fu, R.; Guan, Z. Low-temperature side-chain liquid crystalline polysiloxanes used as stationary phases for capillary gas chromatography. J Chromatogr. A 1989, 483, 394–400. [Google Scholar] [CrossRef]
- Finkelmann, H.; Kock, H.J. Investigations on liquid crystalline polysiloxanes 3. Liquid crystalline elastomers-a new type of liquid crystalline material. Makromol. Rapid Commun. 1981, 2, 317–322. [Google Scholar] [CrossRef]
- Dubois, J.C.; Barny, P.L.; Mauzac, M. Behavior and properties of side chain thermotropic liquid crystal polymers. Acta Polym. Sin. 1997, 48, 47–87. [Google Scholar] [CrossRef]
- Hu, J.S.; Zhang, B.Y.; Zhou, A.J. Side-chain cholesteric liquid crystalline elastomers derived from a mesogenic crosslinking agent: I. Synthesis and mesomorphic properties. Eur. Polym. J. 2006, 42, 2849–2858. [Google Scholar] [CrossRef]
- Yao, W.; Gao, Y.; Zhang, C. A series of novel side chain liquid crystalline polysiloxanes containing cyano- and cholesterol-terminated substituents: Where will the structure-dependence of terminal behavior of the side chain reappear? J. Polym. Sci. Pol. Chem. 2017, 55, 1765–1772. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, Y.; Tang, H. Synthesis and characterization of a graft side-chain liquid crystalline polysiloxane. J. Organomet. Chem. 2015, 775, 50–54. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.Z.; Zheng, J.J. Side-chain cholesteric liquid-crystalline elastomers containing azobenzene derivative as cross-linking agent-synthesis and characterisation. Liq. Cryst. 2018, 45, 1–12. [Google Scholar] [CrossRef]
- Malik, T.M.; Carreau, P.J.; Chapleau, N. Characterization of liquid crystalline polyester polycarbonate blends. Polym. Eng. Sci. 1989, 29, 600–608. [Google Scholar] [CrossRef]
- Chang, J.H.; Choi, B.K.; Kim, J.H. The Effect of Composition on Thermal, Mechanical, and Morphological Properties of Thermotropic Liquid Crystalline Polyester with Alkyl Side-Group and Polycarbonate BIends. Polym. Eng. Sci. 2010, 37, 1564–1571. [Google Scholar] [CrossRef]
- Chan, H.S.; Leng, Y.; Gao, F. Processing of PC/LCP in situ composites by closed-loop injection molding. Compos. Sci. Technol. 2002, 62, 757–765. [Google Scholar] [CrossRef]
- Nanda, M.; Tripathy, D.K. Rheological behavior of chlorosulfonated polyethylene composites: Effect of filler and plasticizer. J. Appl. Polym. Sci. 2012, 126, 46–55. [Google Scholar] [CrossRef]
- Thalib, S.; Huzni, S. The effect of particle compositions on the activation energy of the pa6/bagasse composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 012086. [Google Scholar] [CrossRef] [Green Version]
- Muksing, N.; Nithitanakul, M.; Grady, B.P. Melt rheology and extrudate swell of organobentonite-filled polypropylene nanocomposites. Polym. Test. 2008, 27, 470–479. [Google Scholar] [CrossRef]
- Hemmati, M.; Rahimi, G.H.; Kaganj, A.B. Rheological and Mechanical Characterization of Multi-Walled Carbon Nanotubes/Polypropylene Nanocomposites. J. Macromol. Sci. B. 2008, 47, 1176–1187. [Google Scholar] [CrossRef]












| Samples | LLDPE (g) | MH (g) | PSCTLCP (g) |
|---|---|---|---|
| MH/LLDPE | 40 | 60 | - |
| PSCTLCP-0.6 | 40 | 60 | 0.6 |
| PSCTLCP-1.0 | 40 | 60 | 1.0 |
| PSCTLCP-3.0 | 40 | 60 | 3.0 |
| PSCTLCP-5.0 | 40 | 60 | 5.0 |
| Tg (°C) | Tinitial (°C) | Tm (°C) | Ti (°C) | ΔT (°C) |
|---|---|---|---|---|
| 34.5 | 173.6 | 185.2 | 277.7 | 92.5 |
| γ (s−1) | 200 | 500 | 800 | 1000 | 2000 |
|---|---|---|---|---|---|
| EMH/LLDPE (kJ/mol) | 11.50 | 8.50 | 7.81 | 6.88 | 3.95 |
| EPSCTLCP-1.0 (kJ/mol) | 8.34 | 8.03 | 6.20 | 5.28 | 3.54 |
| R0MH/LLDPE | 0.9919 | 0.9924 | 0.9758 | 0.9759 | 0.9977 |
| R0PSCTLCP-1.0 | 0.9762 | 0.9941 | 0.9922 | 0.9819 | 0.9899 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Cao, B.; Cai, J.; Ye, Z.; Lu, X.; Huang, H.; Liu, S.; Zhao, J. Design and Synthesis of Polysiloxane Based Side Chain Liquid Crystal Polymer for Improving the Processability and Toughness of Magnesium Hydrate/Linear Low-Density Polyethylene Composites. Polymers 2020, 12, 911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040911
Guan X, Cao B, Cai J, Ye Z, Lu X, Huang H, Liu S, Zhao J. Design and Synthesis of Polysiloxane Based Side Chain Liquid Crystal Polymer for Improving the Processability and Toughness of Magnesium Hydrate/Linear Low-Density Polyethylene Composites. Polymers. 2020; 12(4):911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040911
Chicago/Turabian StyleGuan, Xiaoxiao, Bo Cao, Jianan Cai, Zhenxing Ye, Xiang Lu, Haohao Huang, Shumei Liu, and Jianqing Zhao. 2020. "Design and Synthesis of Polysiloxane Based Side Chain Liquid Crystal Polymer for Improving the Processability and Toughness of Magnesium Hydrate/Linear Low-Density Polyethylene Composites" Polymers 12, no. 4: 911. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040911