Systematic Studies on Surface Erosion of Photocrosslinked Polyanhydride Tablets and Data Correlation with Release Kinetic Models
Abstract
:1. Introduction
2. Experimental
2.1. Polymer Synthesis and Characterization
2.2. Fabrication of 3D Printed Molds
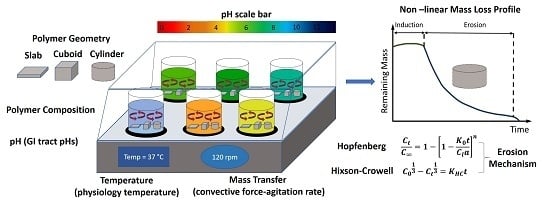
2.3. Experimental Mass Loss Studies under Different Conditions
2.4. Correlation of Erosion Data to Drug Release Kinetic Models
3. Results and Discussion
3.1. Polymer Preparation and Characterization
3.2. A systematic Study on Polymer Mass Loss Profile
3.2.1. The Effect of Crosslinker Ratio (Polymer Composition) on Mass Loss Profile
3.2.2. The Effect of Temperature on Mass Loss
3.2.3. The Effect of Shape/Geometry on Mass Loss Behavior
3.2.4. The Effect of pH on Erosion Profiles
3.2.5. The Effect of Mass Transfer and Model Compound on Erosion Behavior
3.2.6. Pre-Erosion of PAHs to Eliminate the Induction Time
3.2.7. Non-Linear Fitting of Mass Loss Data
3.3. Correlation of Mass Loss Data to Release Kinetic Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Srinath, D.; Lin, S.; Knight, D.K.; Rizkalla, A.S.; Mequanint, K. Fibrous biodegradable l-alanine-based scaffolds for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2014, 8, 578–588. [Google Scholar] [CrossRef]
- Göpferich, A.; Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 2002, 54, 911–931. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric Systems for Controlled Drug Release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Burkersroda FVon Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, H.B.; Chang, J.; Wnek, G.E.; Linhardt, R.J.; Langer, R. Bioerodible polyanhydrides for controlled drug delivery. Biomaterials 1983, 4, 131–133. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Domb, A.; Jain, J.P.; Kumar, N. Handbook of Biodegradable Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 45–75. [Google Scholar] [CrossRef]
- Lesniak, M.S.; Brem, H. Targeted therapy for brain tumours. Nat. Rev. Drug Discov. 2004. [Google Scholar] [CrossRef]
- Poetz, K.L.; Shipp, D.A. Polyanhydrides: Synthesis, Properties, and Applications. Aust. J. Chem. 2016. [Google Scholar] [CrossRef]
- Anseth, K.S.; Shastri, V.R.; Langer, R. Photopolymerizable degradable polyanhydrides with osteocompatibility. Nat. Biotechnol. 1999, 17, 156–159. [Google Scholar] [CrossRef]
- Poshusta, A.K.; Burdick, J.A.; Mortisen, D.J.; Padera, R.F.; Ruehlman, D.; Yaszemski, M.J.; Anseth, K.S. Histocompatibility of photocrosslinked polyanhydrides: A novel in situ forming orthopaedic biomaterial. J. Biomed. Mater. Res. Part A 2003, 64, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Shipp, D.A.; McQuinn, C.W.; Rutherglen, B.G.; McBath, R.A. Elastomeric and degradable polyanhydride network polymers by step-growth thiol-ene photopolymerization. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Rutherglen, B.G.; McBath, R.A.; Huang, Y.L.; Shipp, D.A. Polyanhydride networks from thiol-ene polymerizations. Macromolecules 2010, 43, 10297–10303. [Google Scholar] [CrossRef]
- Poetz, K.L.; Mohammed, H.S.; Snyder, B.L.; Liddil, G.; Samways, D.S.K.; Shipp, D.A. Photopolymerized cross-linked thiol-ene polyanhydrides: Erosion, release, and toxicity studies. Biomacromolecules 2014, 15, 2573–2582. [Google Scholar] [CrossRef]
- Poetz, K.L.; Mohammed, H.S.; Shipp, D.A. Surface eroding, semicrystalline polyanhydrides via thiol-ene “click” photopolymerization. Biomacromolecules 2015, 16, 1650–1659. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Leary, P.E.; Burgess, D.J. Elevated temperature accelerated release testing of PLGA microspheres. J. Control Release 2006, 112, 293–300. [Google Scholar] [CrossRef]
- Aso, Y.; Yoshioka, S.; Li Wan Po, A.; Terao, T. Effect of temperature on mechanisms of drug release and matrix degradation of poly(d,l-lactide) microspheres. J. Control Release 1994, 31, 33–39. [Google Scholar] [CrossRef]
- Ho, K.-L.G.; Pometto, A.L., III; Hinz, P.N.; Ho, K.L.; Pometto, A.L.; Hinz, P.N. Effects of temperature and relative humidity on polylactic acid plastic degradation. J. Polym. Environ. 1999, 7, 83–92. [Google Scholar] [CrossRef]
- Akbari, H.; D’Emanuele, A.; Attwood, D. Effect of geometry on the erosion characteristics of polyanhydride matrices. Int. J. Pharm. 1998. [Google Scholar] [CrossRef]
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.D.; Mitchell, S.A.; Balwinski, K.M. Investigation of the effect of tablet surface area/volume on drug release from hydroxypropylmethylcellulose controlled-release matrix tablets. Drug Dev. Ind. Pharm. 2002, 28, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Ohshima, H.; Kondo, T. Mechanism of hydrolytic degradation of poly(l-lactide) microcapsules: Effects of ph, ionic strength and buffer concentration. J. Microencapsul. 1986, 3, 203–212. [Google Scholar] [CrossRef]
- Leong, K.W.; Brott, B.C.; Langer, R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. J. Biomed. Mater. Res. 1985, 19, 941–955. [Google Scholar] [CrossRef]
- Park, E.S.; Maniar, M.; Shah, J. Effects of model compounds with varying physicochemical properties on erosion of polyanhydride devices. J. Control Release 1996, 40, 111–121. [Google Scholar] [CrossRef]
- Santos, C.A.; Freedman, B.D.; Leach, K.J.; Press, D.L.; Scarpulla, M.; Mathiowitz, E. Poly(fumaric-co-sebacic anhydride): A degradation study as evaluated by FTIR, DSC, GPC and X-ray diffraction. J. Control Release 1999, 60, 11–22. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Shieh, L.; Tamada, J.; Chen, I.; Pang, J.; Domb, A.; Langer, R. Erosion of a new family of biodegradable polyanhydrides. J. Biomed. Mater. Res. 1994, 28, 1465–1475. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release, strategies to modify the drug release from pharmaceutical systems. ResearchGate 2015. [Google Scholar] [CrossRef]
- Fallahi-Sambaran, M.; Salami-Kalajahi, M.; Dehghani, E.; Abbasi, F. Investigation of different core-shell toward Janus morphologies by variation of surfactant and feeding composition: A study on the kinetics of DOX release. Colloid Surf. B 2018. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Mulye, N.V.; Turco, S.J. An examination of assumptions underlying the first-order kinetic model for release of water-soluble drugs from dicalcium phosphate dihydrate matrices. Drug Dev. Ind. Pharm. 1996. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983. [Google Scholar] [CrossRef]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. Fractal kinetics in drug release from finite fractal matrices. J. Chem. Phys. 2003. [Google Scholar] [CrossRef] [Green Version]
- Hixson, A.W.; Crowell, J.H. Dependence of Reaction Velocity upon surface and Agitation: I—Theoretical Consideration. Ind. Eng. Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]
- Katzhendler, I.; Hoffman, A.; Goldberger, A.; Friedman, M. Modeling of Drug Release from Erodible Tablets. J. Pharm. Sci. 1997. [Google Scholar] [CrossRef]
- Langenbucher, F. Letters to the Editor: Linearization of dissolution rate curves by the Weibull distribution. J. Pharm. Pharmacol. 1972. [Google Scholar] [CrossRef] [PubMed]
- Fadda, H.M.; Merchant, H.A.; Arafat, B.T.; Basit, A.W. Physiological bicarbonate buffers: Stabilisation and use as dissolution media for modified release systems. Int. J. Pharm. 2009, 382, 56–60. [Google Scholar] [CrossRef] [PubMed]










| Geometry | Mole Ratio PNA:PETMP:EGDT | Fitting Curve | (R2) |
|---|---|---|---|
 | 100:100:0 | Linear | 0.9579 |
| Quadratic | 0.9995 | ||
| Cubic | 0.9995 | ||
| 100:75:25 | Linear | 0.9448 | |
| Quadratic | 0.9995 | ||
| Cubic | 0.9995 | ||
| 100:50:50 | Linear | 0.9479 | |
| Quadratic | 0.9992 | ||
| Cubic | 0.9996 | ||
| 100:25:75 | Linear | 0.8318 | |
| Quadratic | 0.9673 | ||
| Cubic | 0.9940 | ||
 | 100:100:0 | Linear | 0.9647 |
| Quadratic | 0.9983 | ||
| Cubic | 0.9988 | ||
| 100:75:25 | Linear | 0.9656 | |
| Quadratic | 0.9978 | ||
| Cubic | 0.9993 | ||
| 100:50:50 | Linear | 0.929 | |
| Quadratic | 0.993 | ||
| Cubic | 0.9982 | ||
| 100:25:75 | Linear | 0.8569 | |
| Quadratic | 0.8689 | ||
| Cubic | 0.9998 |
| Model | Mathematical Equation | PNA:PETMP: EGDT | (R2) | Estimated Parameters |
|---|---|---|---|---|
| Zero-order | 100:100:0 | 0.9217 | k0 = −18.34 (−19.55, −17.14) | |
| 100:75:25 | 0.8953 | k0 = -26.5 (−29.1, −23.89) | ||
| 100:50:50 | 0.9226 | k0 = -35.23 (−39.29, −31.18) | ||
| 100:25:75 | 0.9001 | k0 = -45.09 (−55.85, −34.33) | ||
| First-order | 100:100:0 | 0.9370 | k = 0.07648 (0.07046, 0.0824) | |
| 100:75:25 | 0.9451 | k = 0.1346 (0.1222, 0.1471) | ||
| 100:50:50 | 0.9624 | k = 0.1992 (0.1804, 0.218) | ||
| 100:25:75 | 0.8867 | k = 0.458 (0.3527, 0.5633) | ||
| Higuchi | () | 100:100:0 | 0.9494 | KH = 15.54 (14.72, 16.36) |
| 100:75:25 | 0.9770 | KH = 20.36 (19.51, 21.22) | ||
| 100:50:50 | 0.9628 | KH = 25.52 (23.89, 27.15) | ||
| 100:25:75 | 0.2902 | KH = 39.84 (30.07, 49.6) | ||
| Korsmeyer-Peppas (Power Law) | 100:100:0 | 0.8318 | logk = 0.2571 (−0.1998, 0.714) n = 1.367 (0.8176, 1.916) | |
| 100:75:25 | 0.9514 | logk = 0.938 (0.7709, 1.105) n = 0.9001 (0.6661, 1.134) | ||
| 100:50:50 | 0.9555 | logk = 1.259 (1.104, 1.414) n = 0.7061 (0.4262, 0.986) | ||
| 100:25:75 | 0.8341 | logk = 1.695 (0.976, 2.414) n = 0.4218 (−1.968, 2.812) | ||
| Hixson-Crowell | 100:100:0 | 0.9954 | k= 0.01771 (0.01738, 0.0180) | |
| 100:75:25 | 0.9983 | k = 0.02973 (0.02931, 0.0301) | ||
| 100:50:50 | 0.9856 | k = 0.04508 (0.0428, 0.04736) | ||
| 100:25:75 | 0.5750 | k = 0.1009 (0.06404, 0.1377) | ||
| Hopfenberg | () | 100:100:0 | 0.9994 | k = 3.146 (3.113, 3.179) |
| 100:75:25 | 0.9901 | k = 5.366 (5.073, 5.659) | ||
| 100:50:50 | 0.9364 | k = 8.295 (6.895, 9.695) | ||
| 100:25:75 | 0.9508 | k = 18.21 (14, 22.42) | ||
| Weibull | 100:100:0 | 0.9976 | b = 1.294 (1.233, 1.354) a = 37.26 (30.76, 43.76) | |
| 100:75:25 | 0.9926 | b = 1.146 (1.023, 1.268) a = 12.66 (8.982, 16.33) | ||
| 100:50:50 | 0.9591 | b = 0.974 (0.6625, 1.286) a = 5.301 (2.068, 8.534) | ||
| 100:25:75 | 0.1248 | b = 0.6196 (−1.627, 2.866) a = 1.221 (−1.886, 4.328) |
| Model | Mathematical Equation | PNA:PETMP: EGDT | (R2) | Estimated Parameters |
|---|---|---|---|---|
| Zero-order | 100:100:0 | 0.9114 | k0 = -15.53 (−16.76, −14.3) | |
| 100:75:25 | 0.9412 | k0 = -32.12 (−35.7, −28.53) | ||
| 100:50:50 | 0.8874 | k0 = -38.7 (−44.57, −32.84) | ||
| 100:25:75 | 0.6547 | k0 = -124.9 (−265.9, 16.07) | ||
| First-order | 100:100:0 | 0.9690 | k = 0.09395 (0.08832, 0.09958) | |
| 100:75:25 | 0.9990 | k = 0.1163 (0.1144, 0.1181) | ||
| 100:50:50 | 0.9936 | k = 0.1818 (0.1742, 0.1894) | ||
| 100:25:75 | 0.9085 | k = 0.6756 (0.2746, 1.077) | ||
| Higuchi | () | 100:100:0 | 0.9803 | KH = 16.81 (16.19, 17.43) |
| 100:75:25 | 0.9782 | KH = 21.06 (19.65, 22.47) | ||
| 100:50:50 | 0.9805 | KH = 25.89 (24.28, 27.5) | ||
| 100:25:75 | 0.8912 | KH = 54.5 (25.18, 83.81) | ||
| Korsmeyer-Peppas (Power Law) | 100:100:0 | 0.8680 | logk = 0.2705 (−0.1763, 0.7174 n = 1.477 (0.9015, 2.052) | |
| 100:75:25 | 0.7553 | logk = 0.3148 (−0.7788, 1.408) n = 1.89 (-0.08656, 3.867) | ||
| 100:50:50 | 0.7067 | logk = 0.04847 (−2.358, 2.455) n = 2.762 (−2.652, 8.177) | ||
| 100:25:75 | 0.8316 | logk = 0.1069 (−5.915, 6.129) n = 3.501 (−16.52, 23.52) | ||
| Hixson-Crowell | 100:100:0 | 0.9984 | k = 0.02047 (0.02022, 0.02073) | |
| 100:75:25 | 0.9923 | k = 0.0323 (0.03092, 0.03368) | ||
| 100:50:50 | 0.9902 | k = 0.04542 (0.04321, 0.04762) | ||
| 100:25:75 | 0.7724 | k = 0.1758 (0.02395, 0.3277) | ||
| Hopfenberg | () | 100:100:0 | 0.9114 | k = 3.571 (3.288, 3.854) |
| 100:75:25 | 0.9371 | k = 7.452 (6.6, 8.304) | ||
| 100:50:50 | 0.8858 | k = 8.922 (7.564, 10.28) | ||
| 100:25:75 | 0.5071 | k = 31.88 (−6.816, 70.59) | ||
| Weibull | 100:100:0 | 0.9973 | b = 1.188 (1.119, 1.257) a = 21.34 (17.41, 25.27) | |
| 100:75:25 | 0.9990 | b = 0.9593 (0.8992, 1.019) a = 7.856 (6.872, 8.84) | ||
| 100:50:50 | 0.9973 | b = 1.009 (0.8991, 1.12) a = 5.712 (4.485, 6.939) | ||
| 100:25:75 | 0.9796 | b = 0.5083 (−3.196, 4.213) a = 0.8737 (−1.289, 3.037) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraili, A.; Mequanint, K. Systematic Studies on Surface Erosion of Photocrosslinked Polyanhydride Tablets and Data Correlation with Release Kinetic Models. Polymers 2020, 12, 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051105
Geraili A, Mequanint K. Systematic Studies on Surface Erosion of Photocrosslinked Polyanhydride Tablets and Data Correlation with Release Kinetic Models. Polymers. 2020; 12(5):1105. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051105
Chicago/Turabian StyleGeraili, Armin, and Kibret Mequanint. 2020. "Systematic Studies on Surface Erosion of Photocrosslinked Polyanhydride Tablets and Data Correlation with Release Kinetic Models" Polymers 12, no. 5: 1105. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051105