A Novel Synthesis of Poly(Ester-Alt-Selenide)s by Ring-Opening Copolymerization of γ-Selenobutyrolactone and Epoxy Monomer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement
2.3. Typical Polymerization Procedures
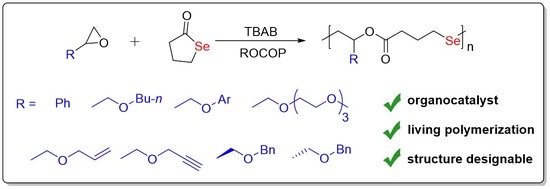
3. Results and Discussion
3.1. Copolymerization of SBL and GPE
3.2. Available Scope of Epoxides
3.3. Preparing of Block Copolymers
3.4. Self-Assembly of Amphiphilic Copolymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boyd, R. Selenium stories. Nat. Chem. 2011, 3, 570. [Google Scholar] [CrossRef] [PubMed]
- Michels, R.; Kato, M.; Heitz, W. Polymeric Reagents. 5. Polymeric Selenium Reagents. Macromol. Chem. Phys. 1976, 177, 2311–2320. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pastor, J.; Barluenga, S.; Winssinger, N. Polymer-supported selenium reagents for organic synthesis. Chem. Commun. 1998, 18, 1947–1948. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium chemistry in stereoselective reactions. Angew. Chem. Int. Ed. 2000, 39, 4189. [Google Scholar]
- Freudendahl, D.M.; Shahzad, S.A.; Wirth, T. Recent Advances in Organoselenium Chemistry. Eur. J. Org. Chem. 2009, 11, 1649–1664. [Google Scholar] [CrossRef]
- Shahzad, S.A.; Wirth, T. Fast Synthesis of Benzofluorenes by Selenium-Mediated Carbocyclizations. Angew. Chem. Int. Ed. 2009, 48, 2588–2591. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Ren, H.F.; Xu, H.P.; Li, Z.B.; Zhang, X. Selenium-containing block copolymers and their oxidation-responsive aggregates. Polym. Chem. 2010, 1, 1609–1614. [Google Scholar] [CrossRef]
- Tan, X.X.; Yu, Y.; Liu, K.; Xu, H.P.; Liu, D.S.; Wang, Z.Q.; Zhang, X. Single-Molecule Force Spectroscopy of Selenium-Containing Amphiphilic Block Copolymer: Toward Disassembling the Polymer Micelles. Langmuir 2012, 28, 9601–9605. [Google Scholar] [CrossRef]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef]
- Jiang, H.N.; Pan, X.Q.; Li, N.; Zhang, Z.B.; Zhu, J.; Zhu, X.L. Selenide-containing high refractive index polymer material with adjustable refractive index and Abbe’s number. React. Funct. Polym. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhang, J.D.; Pan, X.Q.; Zhang, Z.B.; Zhu, J.; Zhu, X.L. Selenide-Containing Polyimides with an Ultrahigh Intrinsic Refractive Index. Polymers 2018, 10, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.J.; Zhou, N.C.; Pan, X.Q.; Zhu, J.; Zhu, X.L. Branched Polystyrene with High Reflex Index Synthesized from Selenium-Mediated Polymerization. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 504–510. [Google Scholar] [CrossRef]
- Lu, W.H.; Pan, X.Q.; Zhang, Z.B.; Zhu, J.; Zhou, N.C.; Zhu, X.L. A degradable cross-linked polymer containing dynamic covalent selenide bond. Polym. Chem. 2017, 8, 3874–3880. [Google Scholar] [CrossRef]
- Zhan, Y.Y.; Zhang, Z.B.; Pan, X.Q.; Zhu, J.; Zhou, N.C.; Zhu, X.L. A cyclic selenium-based reversible addition-fragmentation chain transfer agent mediated polymerization of vinyl acetate. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1656–1663. [Google Scholar] [CrossRef]
- Prince, M.; Bremer, B. Preparation of Hexagonal Polyselenomethylene. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 843–845. [Google Scholar] [CrossRef]
- Zeng, J.D.; Zhu, J.; Zhang, Z.B.; Pan, X.Q.; Zhang, W.; Cheng, Z.P.; Zhu, X.L. New Selenium-Based Iniferter Agent for Living Free Radical Polymerization of Styrene Under UV Irradiation. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2211–2218. [Google Scholar] [CrossRef]
- Ding, C.L.; Fan, C.W.; Jiang, G.Q.; Zhang, J.D.; Li, X.H.; Li, N.; Pan, X.Q.; Zhang, Z.B.; Zhang, W.; Zhu, J.; et al. Diselenide mediated controlled radical polymerization under visible light irradiation: Mechanism investigation and alpha,omega-ditelechelic polymers. Polym. Chem. 2015, 6, 6416–6423. [Google Scholar] [CrossRef]
- Wei, C.; Xu, Y.; Yan, B.K.; Hou, J.Q.; Du, Z.Z.; Lang, M.D. Well-Defined Selenium-Containing Aliphatic Polycarbonates via Lipase-Catalyzed Ring-Opening Polymerization of Selenic Macrocyclic Carbonate Monomer. ACS Macro Lett. 2018, 7, 336–340. [Google Scholar] [CrossRef]
- Yu, L.; Yang, Y.; Du, F.S.; Li, Z.C. ROS-Responsive Chalcogen-Containing Polycarbonates for Photodynamic Therapy. Biomacromolecules 2018, 19, 2182–2193. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, M.; Du, F.S.; Li, Z.C. ROS-responsive poly(ε-caprolactone) with pendent thioether and selenide motifs. Polym. Chem. 2018, 9, 3762–3773. [Google Scholar] [CrossRef]
- Pan, X.Q.; Driessen, F.; Zhu, X.L.; Du Prez, F.E. Selenolactone as a Building Block toward Dynamic Diselenide-Containing Polymer Architectures with Controllable Topology. ACS Macro Lett. 2017, 6, 89–92. [Google Scholar] [CrossRef]
- Wang, C.; An, X.W.; Zhu, J.; Zhang, Z.B.; Pan, X.Q.; Zhu, X.L. Dynamic Diselenide-Containing Polyesters from Alcoholysis/Oxidation of γ-Butyroselenolactone. Polym. Chem. 2018, 9, 4044–4051. [Google Scholar] [CrossRef]
- Zhang, C.J.; Cao, X.H.; Zhang, X.H. Metal-Free Alternating Copolymerization of Nonstrained γ-Selenobutyrolactone with Epoxides for Selenium-Rich Polyesters. Macromolecules 2020, 53, 203–211. [Google Scholar] [CrossRef]
- Sashida, H.; Nakayama, A.; Kaname, M. A New One-Pot Synthetic Method for Selenium-Containing Medium-Sized α,β-Unsaturated Cyclic Ketones. Synthesis 2008, 20, 3229–3236. [Google Scholar] [CrossRef]
- Bakkali-Hassani, C.; Coutouly, C.; Gleede, T.; Vignolle, J.; Wurm, F.R.; Carlotti, S.; Taton, D. Selective Initiation from Unprotected Aminoalcohols for the N-Heterocyclic Carbene-Organocatalyzed Ring-Opening Polymerization of 2-Methyl-N-tosyl Aziridine: Telechelic and Block Copolymer Synthesis. Macromolecules 2018, 51, 2533–2541. [Google Scholar] [CrossRef]
- Nishikubo, T.; Kameyama, A.; Kawakami, S. A novel synthesis of poly(ester-alt-sulfide)s by the ring-opening alternating copolymerization of oxiranes with γ-thiobutyrolactone using quaternary onium salts or crown ether complexes as catalysts. Macromolecules 1998, 31, 4746–4752. [Google Scholar] [CrossRef]
- Cheng, X.X.; Miao, T.F.; Yin, L.; Ji, Y.J.; Li, Y.Y.; Zhang, Z.B.; Zhang, W.; Zhu, X.L. In Situ Controlled Construction of a Hierarchical Supramolecular Chiral Liquid-Crystalline Polymer Assembly. Angew. Chem. Int. Ed. 2020, 59, 2–11. [Google Scholar]
- Ren, H.F.; Wu, Y.T.; Ma, N.; Xu, H.P.; Zhang, X. Side-chain selenium-containing amphiphilic block copolymers: Redox-controlled self-assembly and disassembly. Soft Matter 2012, 8, 1460–1466. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.P.; Wang, Z.Q.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]










| Entry | Catalyst | Solvent | Yield b(%) | Mn,SECcg mol−1 | Ð |
|---|---|---|---|---|---|
| 1 | - | - | - | - | - |
| 2 | TBAC | - | 98 | 4400 | 1.50 |
| 3 | TBPB | - | 98 | 7700 | 1.40 |
| 4 | TBPC | - | 99 | 5200 | 1.47 |
| 5 | TBAB | - | 99 | 8100 | 1.44 |
| 6 | TBAB | DMSO | 60 | 2200 | 1.13 |
| 7 | TBAB | DMF | 74 | 3600 | 1.24 |
| 8 | TBAB | anisole | 98 | 5300 | 1.23 |
| 9 | TBAB | toluene | 98 | 6400 | 1.33 |
| 10 | TBAB | hexane | 98 | 9300 | 1.47 |
| Entry | Oxirane | Yield b (%) | Mn,SECc g mol−1 | Ð |
|---|---|---|---|---|
| 1 | MPE | 97 | 6800 | 1.22 |
| 2 | BGE | 94 | 4100 | 1.16 |
| 3 | SO | 89 | 4500 | 1.22 |
| 4 | AGE | 91 | 4600 | 1.49 |
| 5 | BEA | 92 | 4400 | 3.21 |
| 6 | CHO | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, X.; Zhang, Z.; Zhu, J.; Pan, X.; Zhu, X. A Novel Synthesis of Poly(Ester-Alt-Selenide)s by Ring-Opening Copolymerization of γ-Selenobutyrolactone and Epoxy Monomer. Polymers 2020, 12, 1203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051203
Wang Y, Lin X, Zhang Z, Zhu J, Pan X, Zhu X. A Novel Synthesis of Poly(Ester-Alt-Selenide)s by Ring-Opening Copolymerization of γ-Selenobutyrolactone and Epoxy Monomer. Polymers. 2020; 12(5):1203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051203
Chicago/Turabian StyleWang, Ya’nan, Xiaofang Lin, Zhengbiao Zhang, Jian Zhu, Xiangqiang Pan, and Xiulin Zhu. 2020. "A Novel Synthesis of Poly(Ester-Alt-Selenide)s by Ring-Opening Copolymerization of γ-Selenobutyrolactone and Epoxy Monomer" Polymers 12, no. 5: 1203. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051203