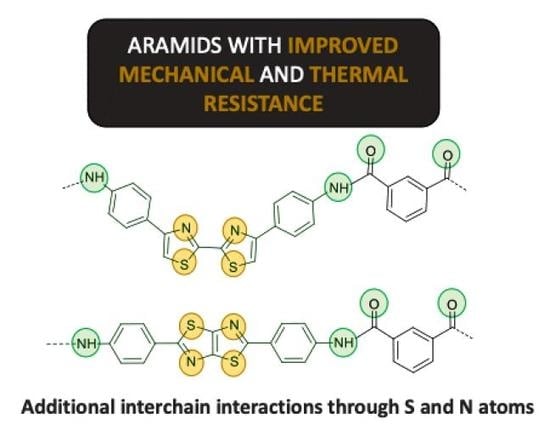
Heteroaromatic Polyamides with Improved Thermal and Mechanical Properties
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Measurements
2.3. Synthesis of Intermediates, Monomers, and Models
2.3.1. Synthesis of Diamine D1 and Model Polyamide M1
2.3.2. Synthesis of Diamine D2 and Model Polyamide M2
2.3.3. Synthesis of Copolyamides CP1 and CP2
3. Results and Discussion
3.1. Synthesis and Characterization of the Monomer, the Model and the Polymers
3.2. Properties of the Polymers
3.2.1. Water Uptake and Solubility
3.2.2. Mechanical Properties of the Copolyamides
3.2.3. Thermal Properties of the Copolyamides
3.2.4. Wide Angle Powder X-ray Scattering
3.2.5. Optical Properties
Luminescence Properties
CIE 1931
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- US Federal Trade Commission (FTC). Rules and regulations under the textile fiber products identification act. Part 303.7 (Generic names and definitions for manufactured fibers); US Federal Trade Commission (FTC): Washington, DC, USA, 2006.
- Reglero Ruiz, J.A.; Trigo-López, M.; García, F.C.; García, J.M. Functional Aromatic Polyamides. Polymers (Basel) 2017, 9, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandaru, A.K.; Chouhan, H.; Bhatnagar, N. High strain rate compression testing of intra-ply and inter-ply hybrid thermoplastic composites reinforced with Kevlar/basalt fibers. Polym. Test. 2020, 84, 106407. [Google Scholar] [CrossRef]
- Oh, J.-H.; Bae, J.-H.; Kim, J.-H.; Lee, C.-S.; Lee, J.-M. Effects of Kevlar pulp on the enhancement of cryogenic mechanical properties of polyurethane foam. Polym. Test. 2019, 80, 106093. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]
- Aharoni, S.M.; Curran, S.A.; Murthy, N.S. Poly(p-phenyleneterephthalamide) and poly(m-phenyleneisophthalamide): Positional isomers with partial miscibility. Macromolecules 1992, 25, 4431–4436. [Google Scholar] [CrossRef]
- Jiang, J.-W.; Pei, X.-L.; Sheng, S.-R.; Wu, X.-Y.; Liu, X.-L.; Song, C.-S. Novel soluble fluorine-containing polyamides derived from 2-(4-trifluoromethylphenoxy)terephthalic acid and trifluoromethyl-substituted aromatic bis(ether amine)s. Polym. Bull. 2011, 67, 263–274. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Huang, Z.-Z.; Song, C.; Tang, C.-C.; Liu, X.-L.; Sheng, S.-R. New fluorinated aromatic polyamides based on N,N -bis(4-carboxyphenyl)-4-trifluoromethylaniline. High Perform. Polym. 2019, 31, 613–622. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, S.; Liu, Y.; Sheng, S.; Huang, Z.; Song, C. Synthesis and Characterization of New Soluble Polyamides from 9,9-Bis[4-(chloroformylphenoxy)phenyl]xanthene and Various Aromatic Diamines. Chin. J. Chem. 2010, 28, 102–110. [Google Scholar] [CrossRef]
- Rafiee, Z.; Mallakpour, S. Synthesis and properties of novel brominated chiral polyamides derived from 5-[4-(2-tetrabromophthalimidylpropanoylamino)benzoylamino]isophthalic acid and aromatic diamines. Polym. Bull. 2016, 73, 1951–1964. [Google Scholar] [CrossRef]
- Yu, G.; Li, B.; Liu, J.; Wu, S.; Tan, H.; Pan, C.; Jian, X. Novel thermally stable and organosoluble aromatic polyamides with main chain phenyl-1,3,5-triazine moieties. Polym. Degrad. Stab. 2012, 97, 1807–1814. [Google Scholar] [CrossRef]
- Chen, J.-C.; Rajendran, K.; Huang, S.-W.; Chang, H.-W. Synthesis and characterization of aromatic polyamides derived from various derivatives of 4,4’-oxydianiline. J. Polym. Res. 2011, 18, 1693–1703. [Google Scholar] [CrossRef]
- Tan, J.; Wang, C.; Peng, W.; Li, G.; Jiang, J.-M. Synthesis, characterization, and properties of novel aromatic polyamides containing phthalazinone moiety. Polym. Bull. 2009, 62, 195–207. [Google Scholar] [CrossRef]
- Bera, D.; Dasgupta, B.; Chatterjee, S.; Maji, S.; Banerjee, S. Synthesis, characterization, and properties of semifluorinated organo-soluble new aromatic polyamides. Polym. Adv. Technol. 2012, 23, 77–84. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, M.; Zhang, Y. Study on synthesis of novel soluble aromatic polyamides with pendant cyano groups. Polym. Bull. 2010, 65, 309–318. [Google Scholar] [CrossRef]
- Zou, F.; Wen, H.; Yan, T.; Cai, M. Synthesis and properties of novel soluble aromatic polyamides containing 4-aryl-2,6-diphenylpyridine moieties and pendant fluorinated phenoxy groups. J. Polym. Res. 2016, 23, 225. [Google Scholar] [CrossRef]
- Carja, I.-D.; Hamciuc, C.; Hamciuc, E.; Vlad-Bubulac, T.; Lisa, G. New highly thermostable aromatic polyamides with pendant phthalonitrile groups. Macromol. Res. 2012, 20, 1011–1020. [Google Scholar] [CrossRef]
- Trigo-López, M.; Pablos, J.L.; García, F.C.; Serna, F.; García, J.M. Functional aramids: Aromatic polyamides with reactive azido and amino groups in the pendant structure. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1469–1477. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wang, W.-L.; Wang, G.-F.; Shi, L.-P.; Gu, M.; Ren, Y.-D.; Hou, L.-F.; He, P.-L.; Zhu, F.-H.; Zhong, X.-G.; et al. Rational Design and Synthesis of 2,2-Bisheterocycle Tandem Derivatives as Non-Nucleoside Hepatitis B Virus Inhibitors. ChemMedChem 2008, 3, 1316–1321. [Google Scholar] [CrossRef]
- Mourer, M.; Psychogios, N.; Laumond, G.; Aubertin, A.-M.; Regnouf-de-Vains, J.-B. Synthesis and anti-HIV evaluation of water-soluble calixarene-based bithiazolyl podands. Bioorg. Med. Chem. 2010, 18, 36–45. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, W.; Tang, J.; Shen, Z. Synthesis of novel copolymers containing tri(ethylene oxide) segments and bithiazole rings on the backbone and magnetic properties of their complexes. Polymer (Guildf) 2006, 47, 6280–6284. [Google Scholar] [CrossRef]
- Geng, J.; Liu, Y.; Li, J.; Yin, G.; Huang, W.; Wang, R.; Quan, Y. A ratiometric fluorescent probe for ferric ion based on a 2,2′-bithiazole derivative and its biological applications. Sens. Actuators B Chem. 2016, 222, 612–617. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Huo, L.; Cui, C.; Wu, Y.; Hou, J.; Li, Y. Poly(thieno[3,2- b ]thiophene- alt -bithiazole): A D–A Copolymer Donor Showing Improved Photovoltaic Performance with Indene-C 60 Bisadduct Acceptor. Macromolecules 2012, 45, 6930–6937. [Google Scholar] [CrossRef]
- Oniwa, K.; Kikuchi, H.; Kanagasekaran, T.; Shimotani, H.; Ikeda, S.; Asao, N.; Yamamoto, Y.; Tanigaki, K.; Jin, T. Biphenyl end-capped bithiazole co-oligomers for high performance organic thin film field effect transistors. Chem. Commun. 2016, 52, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Zahradník, P.; Magdolen, P.; Zahradník, P. Thiazolo[4,5-d]thiazole—A new domain for potential optoelectronic application. Tetrahedron Lett. 2010, 51, 5819–5821. [Google Scholar] [CrossRef]
- Bevk, D.; Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. Thiazolo[5,4-d]thiazoles—Promising building blocks in the synthesis of semiconductors for plastic electronics. RSC Adv. 2013, 3, 11418. [Google Scholar] [CrossRef]
- Kuwabara, J.; Kuramochi, M.; Liu, S.; Yasuda, T.; Kanbara, T. Direct arylation polycondensation for the synthesis of bithiazole-based conjugated polymers and their physical properties. Polym. J. 2017, 49, 123–131. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- Harwood, D.; Aoki, H.; Lee, Y.-D.; Fellers, J.F.; White, J.L. Solution and rheological properties of poly(m-phenyleneisophthalamide) in dimethylacetamide/LiCl. J. Appl. Polym. Sci. 1979, 23, 2155–2168. [Google Scholar] [CrossRef]
- Trigo-López, M.; Miguel-Ortega, Á.; Vallejos, S.; Muñoz, A.; Izquierdo, D.; Colina, Á.; García, F.C.; García, J.M. Intrinsically colored wholly aromatic polyamides (aramids). Dye. Pigment. 2015, 122, 177–183. [Google Scholar] [CrossRef]
- Trigo-López, M.; Barrio-Manso, J.L.; Serna, F.; García, F.C.; García, J.M. Crosslinked Aromatic Polyamides: A Further Step in High-Performance Materials. Macromol. Chem. Phys. 2013, 214, 2223–2231. [Google Scholar] [CrossRef]
- Halasa, A.F.; Wathen, G.D.; Hsu, W.L.; Matrana, B.A.; Massie, J.M. Relationship between interchain spacing of amorphous polymers and blend miscibility as determined by wide-angle X-ray scattering. J. Appl. Polym. Sci. 1991, 43, 183–190. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jung, J.-W.; Kim, S.-S.; Lee, J.-W. m-Aramid Films in Diverse Coagulants. Text. Color. Finish. 2009, 21, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Barrio-Manso, J.L.; Calvo, P.; García, F.C.; Pablos, J.L.; Torroba, T.; García, J.M. Functional fluorescent aramids: Aromatic polyamides containing a dipicolinic acid derivative as luminescent converters and sensory materials for the fluorescence detection and quantification of Cr(vi), Fe(iii) and Cu(ii). Polym. Chem. 2013, 4, 4256. [Google Scholar] [CrossRef]
- Gómez-Valdemoro, A.; Martínez-Máñez, R.; Sancenón, F.; García, F.C.; García, J.M. Functional Aromatic Polyethers: Polymers with Tunable Chromogenic and Fluorogenic Properties. Macromolecules 2010, 43, 7111–7121. [Google Scholar] [CrossRef]
- Miguel-Ortega, Á.; Trigo-López, M.; García, F.C.; Reglero, J.A.; Martínez-Alonso, M.; Espino, G.; García, J.M. Hybrid aramids, Ir(III)-functionalized aromatic polyamides. Eur. Polym. J. 2017, 95, 119–126. [Google Scholar] [CrossRef]
- International Commission on Illumination. The Colour Matching Parameters were Downloaded for Free from the CIE Web Page. Available online: http://files.cie.co.at/204.xls (accessed on 8 February 2020).
- Ohta, N.; Robertson, A.R. CIE Standard Colorimetric System. In Colorimetry. Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 92–96. [Google Scholar]






| Copolymer | ηinha (dL/g) | [η] b (dL/g) | Mw c |
|---|---|---|---|
| CP1 | 0.72 | 0.83 | 3.4 × 104 |
| CP2 | 0.80 | 0.82 | 3.3 × 104 |
| Model or Polymer | Water Uptake a | Solubility b | |||||
|---|---|---|---|---|---|---|---|
| Mass (%) | Water Molecules Per Structural Unit c | DMA | DMF | DMSO | NMP | EtOH, THF, Acetone, CH2Cl2, CHCl3 | |
| M1 | −− | ++ | + | + | ++ | − | |
| M2 | −− | +− | +− | + | + | − | |
| CP1 | 7.1 | 0.98 | ++ | ++ | ++ | ++ | − |
| CP2 | 7.2 | 1.02 | ++ | +− | + | ++ | − |
| Reference d | 5.2 | 1.2 | ++ | ++ | ++ | ++ | − |
| Polymer | Young’s Modulus (GPa) | Tensile Strength (MPa) |
|---|---|---|
| CP1 | 2.2 | 77 |
| CP2 b | 1.9 | 70 |
| Reference a | 1.4 | 63 |
| Polymer | Under Nitrogen | Under Oxygen (Synthetic Air) | LOI c | Tg (°C) | ||||
|---|---|---|---|---|---|---|---|---|
| T5 a (°C) | T10 b (°C) | CR (%) | T5 a (°C) | T10b (°C) | CR (%) | |||
| CP1 | 449 | 470 | 57 | 451 | 483 | 0.7 | 40 | 280 |
| CP2 | 445 | 469 | 58 | 446 | 478 | 0.8 | 41 | 286 |
| Reference d | 432 | 452 | 50 | 419 | 445 | 9 | 38 | 273 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigo-López, M.; Sanjuán, A.M.; Mendía, A.; Muñoz, A.; García, F.C.; García, J.M. Heteroaromatic Polyamides with Improved Thermal and Mechanical Properties. Polymers 2020, 12, 1793. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081793
Trigo-López M, Sanjuán AM, Mendía A, Muñoz A, García FC, García JM. Heteroaromatic Polyamides with Improved Thermal and Mechanical Properties. Polymers. 2020; 12(8):1793. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081793
Chicago/Turabian StyleTrigo-López, Miriam, Ana M. Sanjuán, Aranzazu Mendía, Asunción Muñoz, Félix C. García, and José M. García. 2020. "Heteroaromatic Polyamides with Improved Thermal and Mechanical Properties" Polymers 12, no. 8: 1793. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081793