Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Coating Preparation
2.3. Mesh Coating and Study Groups
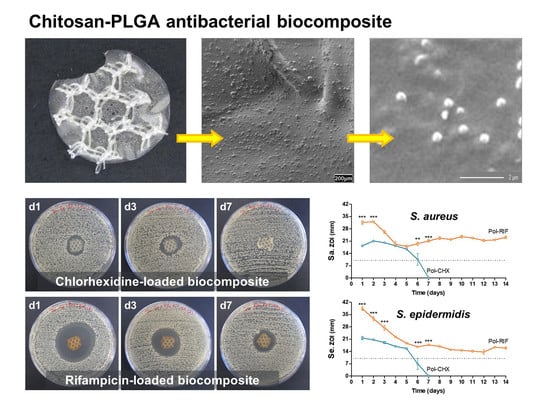
- Control (uncoated PP).
- Pol (PP coated with unloaded polymer).
- Pol-CHX (PP coated with CHX-loaded polymer).
- Pol-RIF (PP coated with RIF-loaded polymer).
2.4. Visualization of the PLGA-Nanoparticles Loaded Biocomposites
2.5. Drug Release
2.6. Cell Viability
2.7. Antibacterial Performance of the Biocomposites
2.7.1. Elaboration of the Bacterial Suspensions
2.7.2. Sequential Agar Well Diffusion Test
2.7.3. Bacterial Adhesion to the Meshes
2.7.4. Turbidimetric Determination of the Bacterial Growth
2.8. Statistical Analysis
3. Results
3.1. Bioactive Polymeric Film
3.2. Release Kinetics of CHX and RIF
3.3. Cell Compatibility of the Biocomposite
3.4. Antibacterial Performance of the Biocomposites
3.4.1. Control of the Bacterial Load
3.4.2. Antibacterial Activity over Time
3.4.3. Prevention of Bacterial Adhesion
3.4.4. Inhibition of Bacterial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalaba, S.; Gerhard, E.; Winder, J.S.; Pauli, E.M.; Haluck, R.S.; Yang, J. Design strategies and applications of biomaterials and devices for hernia repair. Bioact. Mater. 2016, 1, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastegarpour, A.; Cheung, M.; Vardhan, M.; Ibrahim, M.M.; Butler, C.E.; Levinson, H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast. Surg. (Oakv) 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kasiakou, S.K. Mesh-related infections after hernia repair surgery. Clin. Microbiol. Infect. 2005, 11, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, A.; Kallinowski, F.; Köckerling, F. Evidence for replacement of an infected synthetic by a biological mesh in abdominal wall hernia repair. Front. Surg. 2016, 2, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Miguel, I.; Prieto, I.; Albornoz, A.; Sanz, V.; Weis, C.; Turon, P.; Quidant, R. Plasmon-based biofilm inhibition on surgical implants. Nano. Lett. 2019, 19, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Jacombs, A.S.W.; Karatassas, A.; Klosterhalfen, B.; Richter, K.; Patiniott, P.; Hensman, C. Biofilms and effective porosity of hernia mesh: Are they silent assassins? Hernia 2020, 24, 197–204. [Google Scholar] [CrossRef]
- Blatnik, J.A.; Thatiparti, T.R.; Krpata, D.M.; Zuckerman, S.T.; Rosen, M.J.; von Recum, H.A. Infection prevention using affinity polymer-coated, synthetic meshes in a pig hernia model. J. Surg. Res. 2017, 219, 5–10. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Bayon, Y.; Bellón, J.M. Mesh infection and hernia repair: A review. Surg. Infect. 2016, 17, 124–137. [Google Scholar] [CrossRef]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.F.; Richards, R.G.; Eglin, D.; Petter Puchner, A. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nergiz Adıgüzel, E.; Esen, E.; Aylaz, G.; Keskinkılıç Yağız, B.; Kıyan, M.; DoğanÜnal, A.E. Do nano-crystalline silver-coated hernia grafts reduce infection? World J. Surg. 2018, 42, 3537–3542. [Google Scholar] [CrossRef] [PubMed]
- Binnebösel, M.; von Trotha, K.T.; Ricken, C.; Klink, C.D.; Junge, K.; Conze, J.; Jansen, M.; Neumann, U.P.; Lynen Jansen, P. Gentamicin supplemented polyvinylidenfluoride mesh materials enhance tissue integration due to a transcriptionally reduced MMP-2 protein expression. BMC Surg. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Gutiérrez, M.; Olivares, E.; Pascual, G.; Bellón, J.M.; San Román, J. Low-density polypropylene meshes coated with resorbable and biocompatible hydrophilic polymers as controlled release agents of antibiotics. Acta Biomater. 2013, 9, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Scott, J.R.; Novitsky, Y.W. Evaluation of the antimicrobial efficacy of a novel rifampin/minocycline-coated, noncrosslinked porcine acellular dermal matrix compared with uncoated scaffolds for soft tissue repair. Surg. Innov. 2016, 23, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, A.; Cirpanli, Y.; Bilensoy, E.; Yorganci, K.; Caliş, S.; Saribaş, Z.; Kaynaroğlu, V. Antibacterial activity of triclosan chitosan coated graft on hernia graft infection model. Int. J. Pharm. 2009, 381, 214–219. [Google Scholar] [CrossRef]
- Pérez-Köhler, B.; Fernández-Gutiérrez, M.; Pascual, G.; García-Moreno, F.; San Román, J.; Bellón, J.M. In vitro assessment of an antibacterial quaternary ammonium-based polymer loaded with chlorhexidine for the coating of polypropylene prosthetic meshes. Hernia 2016, 20, 869–878. [Google Scholar] [CrossRef]
- Yabanoğlu, H.; Arer, İ.M.; Çalıskan, K. The effect of the use of synthetic mesh soaked in antibiotic solution on the rate of graft infection in ventral hernias: A prospective randomized study. Int. Surg. 2015, 100, 1040–1047. [Google Scholar] [CrossRef]
- Harth, K.C.; Rosen, M.J.; Thatiparti, T.R.; Jacobs, M.R.; Halaweish, I.; Bajaksouzian, S.; Furlan, J.; von Recum, H.A. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: An in vivo study. J. Surg. Res. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Majumder, A.; Neupane, R.; Novitsky, Y.W. Antibiotic coating of hernia meshes: The next step toward preventing mesh infection. Surg. Technol. Int. 2015, 27, 147–153. [Google Scholar]
- Chen, S.; Chen, J.W.; Guo, B.; Xu, C.C. Preoperative antisepsis with chlorhexidine versus povidone-iodine for the prevention of surgical site infection: A systematic review and meta-analysis. World J. Surg. 2020, 44, 1412–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, W.M.; Lin, M.Z.; Song, F.J.; Wang, Y.X.; Xu, K.L.; Huang, J.X.; Fu, J.P.; Peng, Y.Y. Rifampicin as a novel tyrosinase inhibitor: Inhibitory activity and mechanism. Int. J. Biol. Macromol. 2017, 102, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Orelio, C.C.; van Hessen, C.; Sánchez-Manuel, F.J.; Aufenacker, T.J.; Scholten, R.J. Antibiotic prophylaxis for prevention of postoperative wound infection in adults undergoing open elective inguinal or femoral hernia repair. Cochrane Database Syst. Rev. 2020, 4, CD003769. [Google Scholar] [CrossRef] [PubMed]
- Engelsman, A.F.; van der Mei, H.C.; Ploeg, R.J.; Busscher, H.J. The phenomenon of infection with abdominal wall reconstruction. Biomaterials 2007, 28, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Grafmiller, K.T.; Zuckerman, S.T.; Petro, C.; Liu, L.; von Recum, H.A.; Rosen, M.J.; Korley, J.N. Antibiotic-releasing microspheres prevent mesh infection in vivo. J. Surg. Res. 2016, 206, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnebösel, M.; von Trotha, K.T.; Jansen, P.L.; Conze, J.; Neumann, U.P.; Junge, K. Biocompatibility of prosthetic meshes in abdominal surgery. Semin. Immunopathol. 2011, 33, 235–243. [Google Scholar] [CrossRef] [PubMed]
- MacCormick, A.P.; Akoh, J.A. Survey of surgeons regarding prophylactic antibiotic use in inguinal hernia repair. Scand. J. Surg. 2018, 107, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef]
- Guillaume, O.; Lavigne, J.P.; Lefranc, O.; Nottelet, B.; Coudane, J.; Garric, X. New antibiotic-eluting mesh used for soft tissue reinforcement. Acta Biomater. 2011, 7, 3390–3397. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.; Zaat, S.A.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Spångberg, L.S.; Bok, Y.B.; Lee, C.Y.; Kum, K.Y. The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.; Dempsey-Hibbert, N.C.; Whitehead, K.A. Antimicrobial strategies to reduce polymer biomaterial infections and their economic implications and considerations. Int. Biodeter. Biodegr. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Lim, K.S.; Kam, P.C. Chlorhexidine: Pharmacology and clinical applications. Anaesth. Intensive Care 2008, 36, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artsimovitch, I.; Vassylyeva, M.N.; Svetlov, D.; Svetlov, V.; Perederina, A.; Igarashi, N.; Matsugaki, N.; Wakatsuki, S.; Tahirov, T.H.; Vassylyev, D.G. Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell 2005, 122, 351–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Köhler, B.; Linardi, F.; Pascual, G.; Bellón, J.M.; Eglin, D.; Guillaume, O. Efficacy of antimicrobial agents delivered to hernia meshes using an adaptable thermo-responsive hyaluronic acid-based coating. Hernia 2019, in press. [Google Scholar] [CrossRef]
- Bonne, S.; Mazuski, J.E.; Sona, C.; Schallom, M.; Boyle, W.; Buchman, T.G.; Bochicchio, G.V.; Coopersmith, C.M.; Schuerer, D.J. Effectiveness of minocycline and rifampin vs. chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: A before and after trial. J. Am. Coll. Surg. 2015, 221, 739–747. [Google Scholar] [CrossRef]
- Pham, V.T.; Truong, V.K.; Orlowska, A.; Ghanaati, S.; Barbeck, M.; Booms, P.; Fulcher, A.J.; Bhadra, C.M.; Buividas, R.; Baulin, V.; et al. “Race for the surface”: Eukaryotic cells can win. ACS Appl. Mater. Interfaces 2016, 8, 22025–22031. [Google Scholar] [CrossRef] [Green Version]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, present and future of surgical meshes: A review. Membranes (Basel) 2017, 7, 47. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers (Basel) 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Burroughs, L.; Ashraf, W.; Singh, S.; Martínez-Pomares, L.; Bayston, R.; Hook, A.L. Development of dual anti-biofilm and anti-bacterial medical devices. Biomater. Sci. 2020, 8, 3926–3934. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Kotwicka, M.; Urbaniak, P.; Nowak, A.; Skrzypczak-Jankun, E.; Jankun, J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int. J. Mol. Med. 2016, 37, 1594–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]






| Bacteria | Value | Control | Pol | Pol-CHX | Pol-RIF |
|---|---|---|---|---|---|
| Sa | Mean (CFU) | 1.09 × 107 | 3.41 × 109 | 0 | 0 |
| Median (CFU) | 9.15 × 106 | 2.54 × 109 | 0 | 0 | |
| Min. (CFU) | 5.00 × 106 | 2.24 × 108 | 0 | 0 | |
| Max. (CFU) | 2.01 × 107 | 1.23 × 1010 | 0 | 0 | |
| Positive (%) | 100 (6/6) | 100 (6/6) | 0 (0/6) | 0 (0/6) | |
| Se | Mean (CFU) | 1.12 × 105 | 7.08 × 107 | 1.83 × 102 | 0 |
| Median (CFU) | 6.00 × 104 | 6.70 × 107 | 0 | 0 | |
| Min. (CFU) | 2.00 × 104 | 1.98 × 107 | 0 | 0 | |
| Max. (CFU) | 3.60 × 105 | 1.49 × 108 | 1.10 × 103 | 0 | |
| Positive (%) | 100 (6/6) | 100 (6/6) | 16.67 (1/6) | 0 (0/6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Gutiérrez, M.; Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Pascual, G.; García-Fernández, L.; Aguilar, M.R.; Vázquez-Lasa, B.; Bellón, J.M. Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials. Polymers 2020, 12, 1829. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081829
Fernández-Gutiérrez M, Pérez-Köhler B, Benito-Martínez S, García-Moreno F, Pascual G, García-Fernández L, Aguilar MR, Vázquez-Lasa B, Bellón JM. Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials. Polymers. 2020; 12(8):1829. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081829
Chicago/Turabian StyleFernández-Gutiérrez, Mar, Bárbara Pérez-Köhler, Selma Benito-Martínez, Francisca García-Moreno, Gemma Pascual, Luis García-Fernández, María Rosa Aguilar, Blanca Vázquez-Lasa, and Juan Manuel Bellón. 2020. "Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials" Polymers 12, no. 8: 1829. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081829