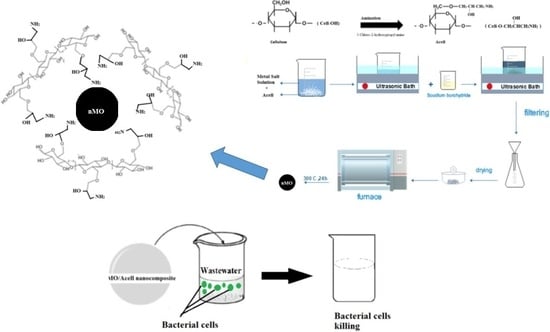
In-Situ Deposition of Metal Oxides Nanoparticles in Cellulose Derivative and Its Utilization for Wastewater Disinfection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aminated Cellulose (Acell)
2.3. In-Situ Deposition of Nanometal Oxides (nMO) into Aminated Cellulose (Acell)
2.4. Characterization
2.5. Release Study of Metal Oxide Nanoparticles
2.6. Antibacterial Activity Evaluation
Disk Diffusion Test Evaluation of the Prepared nMO/Acell Nanocomposites
2.7. Impact of Bacterium–Nanocomposites Interaction Time
2.8. nMO/Acell Nanocomposites Utilization as a Wastewater Disinfectant
3. Results and Discussion
3.1. nMO/Acell Nanocomposites Characterization
3.1.1. FTIR
3.1.2. XRD
3.1.3. SEM and EDX
3.1.4. TEM & SAED
3.2. Release of nMO from Acell into Water
3.3. Disk Diffusion Test Evaluation of the Prepared nMO/Acell
3.4. Contact Time Impact of nMO/Acell on to Antibacterial Activity
3.5. Utilization of nMO/Acell Nanocomposites as a Wastewater Disinfectant
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Obaid, A.Y.; El-Shishtawy, R.M.; Mohamed, S.A. Synthesis of nanocomposites of polypyrrole/carbon nanotubes/silver nano particles and their application in water disinfection. RSC Adv. 2017, 7, 16878–16884. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, M.; Tohya, Y.; Matsubara, K.; Haramoto, E.; Utagawa, E.; Katayama, H. Chlorine inactivation of human norovirus, murine norovirus and poliovirus in drinking water. Lett. Appl. Microbiol. 2010, 51, 119–121. [Google Scholar] [CrossRef]
- Jyoti, K.; Pandit, A. Ozone and cavitation for water disinfection. Biochem. Eng. J. 2004, 18, 9–19. [Google Scholar] [CrossRef]
- Jarvis, P.; Autin, O.; Goslan, E.H.; Hassard, F. Application of ultraviolet light-emitting diodes (UV-LED) to full-scale drinking-water disinfection. Water 2019, 11, 1894. [Google Scholar] [CrossRef] [Green Version]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Env. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Al-Issai, L.; Elshorbagy, W.; Maraqa, M.A.; Hamouda, M.; Soliman, A.M. Use of nanoparticles for the disinfection of desalinated water. Water 2019, 11, 559. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, M.T. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2017, 31, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Lashgari, A.; Ghamami, S.; Golzani, M. Gram-negative and gram-positive bacteria; Antibacterial activity of a clay-TiO2 nanocomposits. Bull. Env. Pharmacol. Life Sci. 2016, 5, 53–59. [Google Scholar]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooda, R.; Sharma, M. Green synthesis, characterization and antibacterial activity of iron oxide nanoparticles. Plant Arch. 2020, 20, 1196–1200. [Google Scholar]
- Gu, Y.; Xiao, F.; Luo, L.; Zhou, X.; Zhou, X.; Li, J.; Li, Z. Bacterial disinfection by CuFe2O4 nanoparticles enhanced by NH2OH: A mechanistic study. Nanomaterials 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, S.; Moeinpour, F. Inactivation of Escherichia coli in water by silver-coated Ni0.5Zn 0.5Fe2O4 magnetic nanocomposite: A Box–Behnken design optimization. Appl. Water Sci. 2019, 9, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hakami, S.M.; Khalil, A.B.; Laoui, T.; Atieh, M.A. Fast disinfection of Escherichia coli bacteria using carbon nanotubes interaction with microwave radiation. Bioinorg. Chem. Appl. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Zhang, C.; Zeng, G.; Gong, J.; Chang, Y.; Jiang, Y. Antibacterial properties and mechanism of graphene oxide-silver nanocomposites as bactericidal agents for water disinfection. Arch. Biochem. Biophys. 2016, 604, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Helen, S.M.; Rani, S.M. Synthesis, characterization and antibacterial activity of nickel chitosan nanoparticles. IOSR-JAC 2016, 9, 8–11. [Google Scholar]
- Pettignano, A.; Charlot, A.; Fleury, E. Solvent-free synthesis of amidated carboxymethyl cellulose derivatives: Effect on the thermal properties. Polymers 2019, 11, 1227. [Google Scholar] [CrossRef] [Green Version]
- Gouda, M.; Hebeish, A.; Al-Omair, M. Development of silver-containing nanocellulosics for effective water disinfection. Cellulose 2014, 21, 1965–1974. [Google Scholar] [CrossRef]
- Eivazihollagh, A.; Bäckström, J.; Dahlström, C.; Carlsson, F.; Ibrahem, I.; Lindman, B.; Edlund, H.; Norgren, M. One-pot synthesis of cellulose-templated copper nanoparticles with antibacterial properties. Mater. Lett. 2017, 187, 170–172. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Zhang, Y.; Shen, M.; Zhang, J. Gas phase synthesis of aminated nanocellulose aerogel for carbon dioxide adsorption. Cellulose 2020. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Luis, V.; Sugam, K.; Emma, M.N.; German, S.-A.; Aji, P.M. In-Situ growth of metal oxide nanoparticles on cellulose nanofibrils for dye removal and antimicrobial applications. ACS Appl. Nano Mater. 2020, 3, 7172–7181. [Google Scholar]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.; Youngblood, J.P. Green one-pot synthesis of surface hydrophobized cellulose nanocrystals in aqueous medium. ACS Sustain. Chem. Eng. 2016, 4, 3927–3938. [Google Scholar] [CrossRef]
- Ross, J.H.; Baker, D.; Coscia, A.T. Some reactions of epichlorohydrin with amines. J. Org. Chem. 1964, 29, 824–826. [Google Scholar] [CrossRef]
- Khalil, M.; Farag, S. Preparation of some cationic starches using the dry process. Starch Stärke 1998, 50, 267–271. [Google Scholar] [CrossRef]
- Gouda, M.; Aljaafari, A.; Al-Fayz, Y.; Boraie, W. Preparation and characterization of some nanometal oxides using microwave technique and their application to cotton fabrics. J. Nanomater. 2015, 2015, 586904. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.W.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Case, C.; Johnson, T. Laboratory Experiments in Microbiology; Benjamin Cummings: San Francisco, CA, USA, 1986. [Google Scholar]
- Abou-Elela, S.; Fawzy, M.; Abdel-Halim, W. Packed bed up-flow anaerobic sludge blanket combined with multistage sand fine roughing filtration for municipal wastewater treatment and reuse. Int. J. Sustain. Dev. Plan. 2013, 8, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of cellulosic fibers by FTIR spectroscopy for their further implementation to building materials. Am. J. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiak, T.; Filipkowska, U.; Brym, S.; Zyśk, M. The use of aminated cotton fibers as an unconventional sorbent to remove anionic dyes from aqueous solutions. Cellulose 2020, 27, 3957–3969. [Google Scholar] [CrossRef] [Green Version]
- Sheena, P.; Priyanka, K.; Sabu, B.; Varghese, T. Effect of calcination temperature on the structural and optical properties of nickel oxide nanoparticles. Nanosyst. Phys. Chem. Math. 2014, 5, 441–449. [Google Scholar]
- Xu, J.; Li, S.; Tan, L.; Kou, B. Enhanced catalytic activity of mesoporous graphitic carbon nitride on thermal decomposition of ammonium perchlorate via copper oxide modification. Mater. Res. Bull. 2017, 93, 352–360. [Google Scholar] [CrossRef]
- Ta, T.K.H.; Trinh, M.T.; Long, N.V.; Nguyen, T.T.M.; Nguyen, T.L.T.; Thuoc, T.L.; Phan, B.T.; Mott, D.; Maenosono, S.; Tran-Van, H. Synthesis and surface functionalization of Fe3O4-SiO2 core-shell nanoparticles with 3-glycidoxypropyltrimethoxysilane and 1, 1′-carbonyldiimidazole for bio-applications. Colloid Surf. A 2016, 504, 376–383. [Google Scholar] [CrossRef]
- Köse, D.A.; Necefoglu, H. Synthesis and characterization of bis(nicotinamide) m-hydroxybenzoate complexes of Co(II), Ni(II), Cu(II) and Zn(II). J. Therm. Anal. Calorim. 2008, 93, 509–514. [Google Scholar] [CrossRef]
- Zaky, M.; El-Sayedl, M.Y.; El-Megharbel, S.M.; Taleb, S.A.; Refat, M.S. Synthesis, chemical structure elucidation and biological studies on the effect of some vital metal ions on lisinopril. J. Mex. Chem. Soc. 2014, 58, 142–151. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, H.; Lue, A.; Hu, K.; Cheng, G.; Zhang, L. Role of sodium zincate on cellulose dissolution in NaOH/urea aqueous solution at low temperature. Carbohydr. Polym. 2011, 83, 1185–1191. [Google Scholar] [CrossRef]
- Zhao, S.-W.; Zheng, M.; Zou, X.H.; Guo, Y.; Pan, Q.J. Self-assembly of hierarchically structured cellulose@zno composite in solid-liquid homogeneous phase: Synthesis, DFT calculations, and enhanced antibacterial activities. ACS Sustain. Chem. Eng. 2017, 5, 6585–6596. [Google Scholar] [CrossRef]
- Si-Wei, Z.; Chong-Rui, G.; Ying-Zhu, H.; Yuan-Ru, G.; Qing-Jiang, P. The preparation and antibacterial activity of cellulose/ZnO composite: Review. Open Chem. 2018, 16, 9–20. [Google Scholar]
- Brewerp, D.G.; Wong, T.T.; Sears, M.C. The nature of the coordination bond in metal complexes of substituted pyridine derivatives. III. 4-Methylpyridine complexes of some divalent transition metal ions. Can. J. Chem. 1968, 46, 3137–3141. [Google Scholar] [CrossRef]
- Diva, T.N.; Zare, K.; Taleshi, F.; Yousefi, M. Synthesis, characterization, and application of nickel oxide/CNT nanocomposites to remove Pb2+ from aqueous solution. J. Nanostruct. Chem. 2017, 7, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Wan Ishak, W.H.; Ahmad, I.; Ramli, S.; Mohd Amin, M.C.I. Gamma irradiation-assisted synthesis of cellulose nanocrystal-reinforced gelatin hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, N.; Srivastava, P. Realizing NiO nanocrystals from a simple chemical method. Bull. Mater. Sci. 2010, 33, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Batool, M.; Qureshi, M.Z.; Hashmi, F.; Mehboob, N.; Shah, A.S. Congo red azo dye removal and study of its kinetics by aloe vera mediated copper oxide nanoparticles. Indones. J. Chem. 2019, 19, 626–637. [Google Scholar] [CrossRef]
- Kumar, P.; Nene, A.G.; Punia, S.; Kumar, M.; Abbas, Z.; Thakral, F.; Tuli, H.S. Synthesis, characterization and antibacterial activity of CuO nanoparticles. Int. J. App. Pharm. 2020, 12, 17–20. [Google Scholar] [CrossRef]
- Noval, V.E.; Carriazo, J.G. Fe3O4-TiO2 and Fe3O4-SiO2 core-shell powders synthesized from industrially processed magnetite (Fe3O4) microparticles. Mater. Res. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2003, 40, 4346–4352. [Google Scholar] [CrossRef]
- Porter, A.E.; Muller, K.; Skepper, J.; Midgley, P.; Welland, M. Uptake of C60 by human monocyte macrophages, its localization and implications for toxicity: Studied by high resolution electron microscopy and electron tomography. Acta Biomater. 2006, 2, 409–419. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Nakamura, N.; Komine, T. Continuous-sterilization system that uses photosemiconductor powders. Appl. Environ. Microbiol. 1988, 54, 1330–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B Biol. 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Ravichandran, K.; Rathi, R.; Baneto, M.; Karthika, K.; Rajkumar, P.; Sakthivel, B.; Damodaran, R. Effect of Fe+ F doping on the antibacterial activity of ZnO powder. Ceram. Int. 2015, 41, 3390–3395. [Google Scholar] [CrossRef]
- Budde, K.; Ratnam, K.V.; Babu, K.S. Preparation and antimicrobial activity evaluation of metal oxide nanoparticles of copper, nickel and zinc, nickel and zinc. SSRN Electron. 2020, 10, 89–107. [Google Scholar]
- Yu-sen, E.L.; Vidic, R.D.; Stout, J.E.; McCartney, C.A.; Victor, L.Y. Inactivation of Mycobacterium avium by copper and silver ions. Water Res. 1998, 32, 1997–2000. [Google Scholar]
- Lin, Y.S.E.; Vidic, R.D.; Stout, J.E.; Victor, L.Y. Individual and combined effects of copper and silver ions on inactivation of Legionella pneumophila. Water Res. 1996, 30, 1905–1913. [Google Scholar] [CrossRef]
- Blanc, D.; Carrara, P.; Zanetti, G.; Francioli, P. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: Seven years of experience in a university teaching hospital. J. Hosp. Infect. 2005, 60, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M. Nano-zirconium oxide and nano-silver oxide/cotton gauze fabrics for antimicrobial and wound healing acceleration. J. Ind. Text. 2012, 41, 222–240. [Google Scholar] [CrossRef]






| nMO/Acell Type | Clear Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| Gram-Negative Bacteria | Gram-Positive Bacteria | |||
| Escherichia coli | Salmonella typhi | Staphylococcus aureus | Enterococcus faecalis | |
| nFe3O4/Acell | 18 mm | 16 mm | 24 mm | 22 mm |
| nCuO/Acell | 12 mm | 10 mm | 20 mm | 17 mm |
| nNiO/Acell | 5 mm | 5 mm | 10 mm | 8 mm |
| Contact Time (mins) | Bacterial Reduction (R%) and (CFU/100 mL) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nF3O4/Acell | nCuO/Acell | nNiO/Acell | ||||||||||||||||||||||
| S. aureus | E. faecalis | E. coli | S. typhi | S. aureus | E. faecalis | E. coli | S. typhi | S. aureus | E. faecalis | E. coli | S. typhi | |||||||||||||
| CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | CFU | R% | |
| * 𝐶0 | 2.1 × 104 | 32.3 | 2.5 × 104 | 30.6 | 3.6 × 104 | 21.7 | 5.3 × 103 | 17.2 | 2.3 × 104 | 25.8 | 2.8 × 104 | 22.0 | 3.7 × 104 | 19.6 | 5.5 × 103 | 14.1 | 2.5 × 104 | 19.4 | 3.0 × 104 | 16.6 | 3.9 × 104 | 15.2 | 5.6 × 103 | 12.5 |
| 15 mins | 1.5 × 104 | 51.6 | 1.9 × 104 | 47.2 | 3.1 × 104 | 32.6 | 4.5 × 103 | 29.7 | 1.7 × 104 | 45.1 | 2.1 × 104 | 41.6 | 3.3 × 104 | 26.1 | 4.8 × 103 | 25.0 | 1.9 × 104 | 38.7 | 2.6 × 104 | 27.8 | 3.5 × 104 | 23.9 | 5.1 × 103 | 20.3 |
| 30 mins | 1.2 × 104 | 61.3 | 1.6 × 104 | 55.6 | 2.7 × 104 | 41.3 | 3.8 × 103 | 40.6 | 1.4 × 104 | 54.8 | 1.9 × 104 | 47.2 | 2.9 × 104 | 36.9 | 4.2 × 103 | 34.4 | 1.6 × 104 | 48.4 | 2.1 × 104 | 41.7 | 3.1 × 104 | 32.6 | 4.5 × 103 | 29.7 |
| 60 mins | 7.2 × 103 | 76.7 | 9.7 × 103 | 73.0 | 1.6 × 104 | 65.2 | 2.6 × 103 | 59.4 | 9.7 × 103 | 68.7 | 1.3 × 104 | 63.9 | 1.8 × 104 | 60.9 | 2.9 × 103 | 54.7 | 1.3 × 104 | 58.1 | 1.6 × 104 | 55.5 | 2.1 × 104 | 54.3 | 3.2 × 103 | 50.0 |
| 120 mins | 3.1 × 103 | 90.0 | 4.3 × 103 | 88.0 | 9.1 × 103 | 80.2 | 1.2 × 103 | 80.0 | 6.0 × 103 | 80.6 | 8.4 × 103 | 76.6 | 1.2 × 104 | 73.9 | 1.6 × 103 | 75.0 | 7.7 × 103 | 75.2 | 1.1 × 104 | 69.4 | 1.4 × 104 | 69.6 | 1.9 × 103 | 70.3 |
| 180 mins | 6.2 × 102 | 98.0 | 9.2 × 102 | 97.0 | 5.4 × 103 | 88.2 | 7.8 × 102 | 87.8 | 2.3 × 103 | 92.5 | 4.1 × 103 | 88.6 | 8.3 × 103 | 82 | 1.3 × 103 | 79.7 | 3.5 × 103 | 88.7 | 5.3 × 103 | 85.3 | 1.1 × 104 | 76.0 | 1.4 × 103 | 78.1 |
| 1440 mins | 6.0 × 10 | 99.8 | 3.3 × 102 | 99.0 | 1.5 × 103 | 96.7 | 2.5 × 102 | 96.1 | 1.6 × 103 | 94.8 | 2.0 × 103 | 94.4 | 3.7 × 103 | 92 | 5.4 × 102 | 91.5 | 2.3 × 103 | 92.5 | 3.2 × 103 | 91.1 | 6.9 × 103 | 85.0 | 9.6 × 102 | 85.0 |
| Parameters | Raw Wastewater | Treated Effluent | ||
|---|---|---|---|---|
| nFe3O4/Acell | nCuO/Acell | nNiO/Acell | ||
| Total coliform (MPN-index/100 mL) | 2.8 × 107 | 1.1 × 103 | 2.2 × 103 | 3.7 × 103 |
| Fecal coliform (MPN-index/100 mL) | 1.5 × 107 | 2.8 × 102 | 3.3 × 102 | 3.1 × 103 |
| Time | Control | nFe3O4/Acell | nCuO/Acell | nNiO/Acell | ||||
|---|---|---|---|---|---|---|---|---|
| TC | Fc | TC | Fc | TC | Fc | TC | Fc | |
| Zero time | 4.1 × 103 | 1.8 × 102 | 4.1 × 103 | 1.8 × 102 | 4.1 × 103 | 4.1 × 103 | 4.1 × 103 | 4.1 × 103 |
| 2 mins | 4.6 × 103 | 1.7 × 102 | 1.1 × 103 | 1.4 × 102 | 2.2 × 103 | 3.3 × 102 | 3.7 × 103 | 3.1 × 103 |
| 5 mins | 5.1 × 103 | 2.1 × 102 | 7.5 × 102 | 1.1 × 102 | 1.0 × 103 | 2.5 × 102 | 1.8 × 103 | 4.4 × 102 |
| 10 mins | 5.7 × 103 | 2.6 × 102 | 1.8 × 10 | 1.5 × 10 | 6.5 × 102 | 5.4 × 10 | 8.2 × 102 | 7.3 × 10 |
| 15 mins | 6.2 × 103 | 3.1 × 102 | 1.5 × 10 | 1.0 × 10 | 2.2 × 102 | 3.2 × 10 | 5.3 × 102 | 5.2 × 10 |
| 20 mins | 6.4 × 103 | 4.1 × 102 | 1.1 × 10 | 5.3 | 6.2 × 10 | 8.8 | 1.3 × 102 | 1.1 × 10 |
| 30 mins | 6.8 × 103 | 5.8 × 102 | 1.0 × 10 | 5 | 3.1 × 10 | 6.7 | 4.7 × 10 | 9.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.; Al-Bokheet, W.; Al-Omair, M. In-Situ Deposition of Metal Oxides Nanoparticles in Cellulose Derivative and Its Utilization for Wastewater Disinfection. Polymers 2020, 12, 1834. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081834
Gouda M, Al-Bokheet W, Al-Omair M. In-Situ Deposition of Metal Oxides Nanoparticles in Cellulose Derivative and Its Utilization for Wastewater Disinfection. Polymers. 2020; 12(8):1834. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081834
Chicago/Turabian StyleGouda, Mohamed, Wedad Al-Bokheet, and Mohamed Al-Omair. 2020. "In-Situ Deposition of Metal Oxides Nanoparticles in Cellulose Derivative and Its Utilization for Wastewater Disinfection" Polymers 12, no. 8: 1834. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081834