Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Natural and Accelerated Ageing Procedures
- -
- Natural Ageing (NA): In laboratory conditions, at 20 ± 2 °C and 55 ± 3% relative humidity for a total ageing time of 1488 h;
- -
- High Temperatures (HT) at 100 °C and 55% relative humidity for a total ageing time of 1488 h;
- -
- -
- Photo oxidation by exposure (UV) to an OSRAM Ultra-Vitalux® solar lamp (300 W, 230 V) in a ventilated chamber (45% RH; 31–32 °C), for a total ageing time of 1488 h. The wavelength of the light emitted was from 280 to 2000 nm (13.6 W in the range 315–400 nm, 3.0 W in the range 280–315).
2.3. Analytical Methods
2.3.1. Characterisation of Starting Materials
2.3.2. Assessment of Morphological and Chemical–Physical Variations after Natural and Accelerated Ageing Procedures
2.3.3. Assessment of the Thermal Behaviour before and after Ageing Procedures
3. Results and Discussion
3.1. Characterisation of Starting Materials
- -
- The narrow and sharp peaks at 2954 cm−1 and 2869 cm−1 are associated with the asymmetric and symmetrical stretching of the methyl -CH3 group, respectively, due both to the acrylic dispersion and the wax emulsion;
- -
- Similarly, the narrow and sharp absorptions at 2917 and 2849 cm−1 related to the asymmetric and symmetric stretching of the methylene group are linked to acrylic copolymers and wax;
- -
- The C=O stretching band at 1726 cm−1 and C-O-C stretching band at 1144 cm−1 of the ester absorptions are linked to the presence of the acrylic emulsion; they may also indicate the co-presence of a natural wax in the formulation;
- -
- The peaks at 1450 cm−1 and 1461 cm−1 can be associated with -CH2 bending vibration, together with 1385 cm−1, which refers to out-plane CH bending;
- -
- The absorptions at 1240 and 1167 cm−1 refer to the stretching of the group C-O and C-C;
- -
- Two sharp rocking vibrations at 734 and 723 cm−1 of methylene groups characteristic of a compound with a long aliphatic chain, such as a wax;
3.2. Edelwachs and Mock-Ups Post Treatments
3.2.1. Morphological and Colorimetric Changes
- -
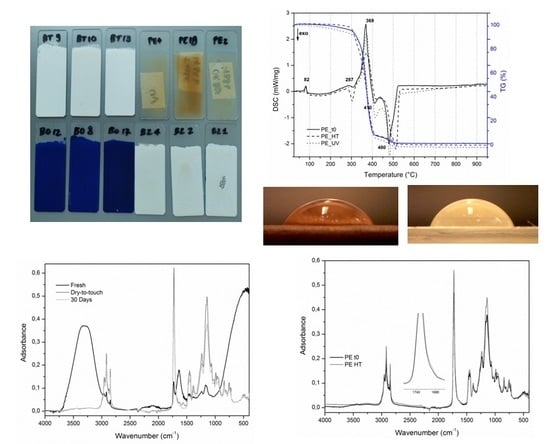
- Natural Ageing: The mock-ups left at laboratory conditions did not show any substantial macro and micro-morphological variations. An exception is by the mock-up containing zinc white, which appeared slightly more opaque compared to the un-aged one. As concerns the colorimetric measurements, the variation in terms of L*, a*, b* was lower than 5, since the values of the ΔE are less than 1 for pigmented mock-ups and 3 for the Edelwachs film (Table 4), mainly due to the negative variation of the b* parameter.
- -
- High Temperature: Microscopic observations of the surfaces of titanium and zinc white evidenced the formation of translucent spots, as depicted in Figure 4. These spots, which appeared to have a yellow-greenish fluorescence under UV-light observation (Figure 4), can be attributed to the migration of the surfactants from the paint bulk to the film surface. As seen before, FT-IR-ATR and Py-GC-MS detected the presence of several additives, among them surfactants. As widely discussed in the literature, accelerated ageing treatments (also with high temperature) can promote the migration of surfactants, which are then visible on the painted surfaces [47].
- -
- UV ageing: Any relevant morphological change was observed though macro and microscopic observations.
- -
- High moisture ageing: Under the conditions used, no substantial morphological differences were observed, nor when using UV light. Besides, colour measurements did not register any relevant colour modification in terms of L*a*b*, as reported in Table 4.
3.2.2. Chemical Changes
- -
- Natural ageingAny chemical variation was detected for Edelwachs film since IR spectra do not show any relevant modification in their profile. The same consideration can be made for the pigmented mock-ups.
- -
- High temperatures
- -
- UV ageingIn the unpigmented Edelwachs film subjected to UV exposure, the appearance of a small peak at 1773 cm−1 was noted, probably indicating the formation of a γ-lactone structure [1,2,3,14].Very interesting is in this sample a decrease of the b* parameter (reduction of the yellow component of the color) after the UV exposure, in addition to a general and significant total colour variation. However, the presence of γ-lactones, generally associated with a yellowing of the polymer matrices, in this case was not depicted.After 60 days of UV ageing, the mock-ups containing ultramarine blue underwent the most evident degradation processes. In particular, a strong IR intensity decrease of the vibrations referring to the binding media and the contribution of -CH3 stretching (2900–2800 cm−1), C=O stretching (1726 cm−1) e C-O-C peak (Figure 7) were registered. At the same time, a broadening of the C=O peak and the formation of a small peak at 1780 cm−1 were registered. As pointed out in similar studies, this variation may be associated with the loss of low molecular weight compounds because of the cross-linking and the fragmentation of side chains [2] and the formation of γ-lactones, as seen for unpigmented Edelwachs film.
- -
- HR ageingAfter the ageing procedures using 85% HR, unpigmented and pigmented mock-ups did not show any relevant IR modifications.
3.2.3. Wettability Changes
3.2.4. General Considerations on the Effects of the Ageing Procedures
3.3. Evaluation of the Thermal Behaviour
- -
- The glass transition temperature (Tg) occurs at about 36 °C: This value is probably due to the influence of the wax components mixed with acrylic monomers.
- -
- The endothermic process at 82 °C refers to a solid–liquid phase change (melting) of the waxy components, where no mass variations are involved.
- -
- In the range of 300–500 °C, an endothermic (369 °C) and two exothermic processes (410 and 480 °C) were registered: The first is due to the melting of the acrylates and the two remaining exothermic peaks to their decomposition (combustion). Generally, the position of these decomposition processes may shift depending on the monomers involved: In this case, they depend on the overall contributions of the four monomers (MA, MMA, BMA and nBA), identified by Py-GC-MS.
- -
- At 287 °C, it is also possible to detect a not well resolved exothermic peak, likely associated with the decomposition of the waxy components.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Pintus, V.; Schreiner, M. Characterization and identification of acrylic binding media: Influence of UV light on the ageing process. Anal. Bioanal. Chem. 2011, 399, 2961–2976. [Google Scholar] [CrossRef] [PubMed]
- Pintus, V.; Ploeger, R.; Chiantore, O.; Wei, S.; Schreiner, M. Thermal analysis of the interaction of inorganic pigments with p(nBA/MMA) acrylic emulsion before and after UV ageing. J. Therm. Anal. Calorim. 2013, 114, 33–43. [Google Scholar] [CrossRef]
- Melchiorre Di Crescenzo, M.; Zendri, E.; Sánchez-Pons, M.; Fuster-López, L.; Yusá-Marco, D.J. The use of waterborne paints in contemporary murals: Comparing the stability of vinyl, acrylic and styrene-acrylic formulations to outdoor weathering conditions. Polym. Degrad. Stab. 2014, 107. [Google Scholar] [CrossRef]
- Owen, L.; Ploeger, R.; Murray, A. The effects of water exposure on surface characteristics of acrylic emulsion paints. J. Can. Assoc. Conserv. = J. l’Association Can. Conserv. Restaur. 2004, 29, 8–25. [Google Scholar]
- Krysztafkiewicz, A.; Jesionowski, T.; Dec, A. Modified titanium white—Characteristics and application. Physicochem. Probl. Miner. Process. 2001, 35, 195–205. [Google Scholar]
- Ormsby, B.; Smithen, P.; Learner, T. Translating research into practice: Evaluating the surface cleaning treatment of an acrylic emulsion painting by Jeremy Moon. In Contemporary Collections: Preprints from the AICCM National Conference 17th–19th October 2007, Brisbane; AICCM: Oklahoma City, OK, USA, 2007. [Google Scholar]
- Brunelli, A.; Badetti, E.; Basei, G.; Izzo, F.C.; Hristozov, D.; Marcomini, A. Effects of organic modifiers on the colloidal stability of TiO2 nanoparticles. A methodological approach for NPs categorization by multivariate statistical analysis. NanoImpact 2018. [Google Scholar] [CrossRef]
- Papliaka, Z.E.; Andrikopoulos, K.S.; Varella, E.A. Study of the stability of a series of synthetic colorants applied with styrene-acrylic copolymer, widely used in contemporary paintings, concerning the effects of accelerated ageing. J. Cult. Herit. 2010. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part II: Photo-oxidative and salt-induced weathering of acrylic-silicone mixtures. Polym. Degrad. Stab. 2007. [Google Scholar] [CrossRef]
- Charola, A.E.; Price, C.A. Stone Conservation: An Overview of Current Research; Getty Conservation Institute: Marina del Rey, CA, USA, 1998; Volume 37, ISBN 9781606060469. [Google Scholar]
- Chiantore, O.; Scalarone, D.; Learner, T. Characterization of artists’ acrylic emulsion paints. Int. J. Polym. Anal. Charact. 2003, 8. [Google Scholar] [CrossRef]
- Biscontin, G.; Zendri, E.; Schionato, A. Protettivi acrilici nella conservazione della pietra. TT—Protective acrylics for stone conservation. Mater. Strutt. 1991, 3, 95–110. [Google Scholar]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer (Guildf.) 2000. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. UV ageing studies: Evaluation of lightfastness declarations of commercial acrylic paints. Anal. Bioanal. Chem. 2012, 402, 1567–1584. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of behaviour on simulated daylight ageing of artists’ acrylic and poly(vinyl acetate) paint films. Anal. Bioanal. Chem. 2011, 399, 2921–2937. [Google Scholar] [CrossRef]
- Kampasakali, E.; Ormsby, B.; Cosentino, A.; Miliani, C.; Learner, T. A preliminary evaluation of the surfaces of acrylic emulsion paint films and the effects of wet-cleaning treatment by atomic force microscopy (AFM). Stud. Conserv. 2011. [Google Scholar] [CrossRef]
- Willneff, E.A.; Schroeder, S.L.M.; Ormsby, B.A. Spectroscopic techniques and the conservation of artists’ acrylic emulsion paints. Herit. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- TiO2 Impact on Paint Weather Resistance—Coatings World. Available online: https://www.coatingsworld.com/issues/2017-09-01/view_features/tio2-impact-on-paint-weather-resistance/ (accessed on 6 August 2020).
- Methods for Field Measuring Coating Thickness. Available online: https://www.tpr2.com/pdf/TPR2_Coating_Measurement_Pocedure.pdf (accessed on 18 August 2020).
- Feller, R.L. Accelerated Aging: Photochemical and Thermal Aspects; Research i; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; ISBN 0-89236-125-5. [Google Scholar]
- Wojciechowski, K.; Zukowska, G.Z.; Korczagin, I.; Malanowski, P. Effect of TiO2 on UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coatings 2015, 85. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Thermal-ageing of paraloid acrylic protective polymers. Polymer (Guildf.) 2000, 41. [Google Scholar] [CrossRef]
- O’Brien, F.E.M. The control of humidity by saturated salt solutions. J. Sci. Instrum. 1948. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977. [Google Scholar] [CrossRef]
- Izzo, F.C.; Capogrosso, V.; Gironda, M.; Alberti, R.; Mazzei, C.; Nodari, L.; Gambirasi, A.; Zendri, E.; Nevin, A. Multi-analytical non-invasive study of modern yellow paints from postwar Italian paintings from the International Gallery of Modern Art Cà Pesaro, Venice. X Ray Spectrom. 2015. [Google Scholar] [CrossRef]
- Izzo, F.C.; Carrieri, A.; Bartolozzi, G.; Keulen, H.V.; Lorenzon, I.; Balliana, E.; Cucci, C.; Grazzi, F.; Picollo, M. Elucidating the composition and the state of conservation of nitrocellulose-based animation cells by means of non-invasive and micro-destructive techniques. J. Cult. Herit. 2019, 35. [Google Scholar] [CrossRef]
- Izzo, F.C.; Zanin, C.; van Keulen, H.; da Roit, C. From pigments to paints: Studying original materials from the atelier of the artist Mariano Fortuny y Madrazo. Int. J. Conserv. Sci. 2017, 8, 547–564. [Google Scholar]
- Izzo, F.C.; Vitale, V.; Fabbro, C.; Van Keulen, H. Multi-analytical investigation on felt-tip pen inks: Formulation and preliminary photo-degradation study. Microchem. J. 2016, 124. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Pinton, F.; Zendri, E. A preliminary study of the composition of commercial oil, acrylic and vinyl paints and their behaviour after accelerated ageing conditions. Conserv. Sci. Cult. Herit. 2014, 14, 353–369. [Google Scholar]
- van Keulen, H.; Schilling, M. AMDIS & EXCEL: A Powerful Combination for Evaluating THM-Py-GC/MS Results from European Lacquers. Stud. Conserv. 2019. [Google Scholar] [CrossRef]
- UNI EN 15802:2010. UNI EN 158022010 Conserv. dei beni Cult.—Metod. di prova—Determ. dell’angolo di Contatto Statico; UNI: Milan, Italy, 2010. [Google Scholar]
- Sanmartín, P.; Cappitelli, F. Evaluation of accelerated ageing tests for metallic and non-metallic graffiti paints applied to stone. Coatings 2017, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Costantini, R.; Berghe, I.V.; Izzo, F.C. New insights into the fading problems of safflower red dyed textiles through a HPLC-PDA and colorimetric study. J. Cult. Herit. 2019. [Google Scholar] [CrossRef]
- Zendri, E.; Falchi, L.; Izzo, F.C.; Morabito, Z.M.; Driussi, G. A review of common NDTs in the monitoring and preservation of historical architectural surfaces. Int. J. Archit. Herit. 2017. [Google Scholar] [CrossRef]
- Izzo, F.C.; Zendri, E.; Biscontin, G.; Balliana, E. TG-DSC analysis applied to contemporary oil paints. J. Therm. Anal. Calorim. 2011, 104. [Google Scholar] [CrossRef]
- Topçuoǧlu, Ö.; Altinkaya, S.A.; Balköse, D. Characterization of waterborne acrylic based paint films and measurement of their water vapor permeabilities. Prog. Org. Coatings 2006, 56. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, K.; Skowera, E.; Pietniewicz, E.; Zukowska, G.Z.; Van Der Ven, L.G.J.; Korczagin, I.; Malanowski, P. UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coatings 2014, 77. [Google Scholar] [CrossRef]
- Daimon, H.; Okitsu, H.; Kumanotani, J. Glass transition behaviors of random and block copolymers and polymer blends of styrene and cyclododecyl acrylate. I. Glass transition temperatures. Polym. J. 1975, 7. [Google Scholar] [CrossRef]
- Musser, B.J.; Kilpatrick, P.K. Molecular characterization of wax isolated from a variety of crude oils. Energy Fuels 1998, 12. [Google Scholar] [CrossRef]
- Binks, B.P.; Rocher, A. Effects of temperature on water-in-oil emulsions stabilised solely by wax microparticles. J. Colloid Interface Sci. 2009, 335. [Google Scholar] [CrossRef] [PubMed]
- Japper-Jaafar, A.; Bhaskoro, P.T.; Mior, Z.S. A new perspective on the measurements of wax appearance temperature: Comparison between DSC, thermomicroscopy and rheometry and the cooling rate effects. J. Pet. Sci. Eng. 2016, 147. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Comite, V.; Ortenzi, M.A.; Fermo, P.; Gulotta, D.; Cappelletti, G. Towards novel fluorinated methacrylic coatings for cultural heritage: A combined polymers and surfaces chemistry study. Polymers 2019, 11, 1190. [Google Scholar] [CrossRef] [Green Version]
- Beltran, V.; Salvadó, N.; Butí, S.; Cinque, G. Micro infrared spectroscopy discrimination capability of compounds in complex matrices of thin layers in real sample coatings from artworks. Microchem. J. 2014, 118, 115–123. [Google Scholar] [CrossRef] [Green Version]
- CARNAUBA WAX—FTIR Spectrum—SpectraBase. Available online: https://spectrabase.com/spectrum/Jr07zupmVuW (accessed on 6 August 2020).
- Ziraldo, I.; Watts, K.; Luk, A.; Lagalante, A.F.; Wolbers, R.C. The influence of temperature and humidity on swelling and surfactant migration in acrylic emulsion paint films. Stud. Conserv. 2016. [Google Scholar] [CrossRef]
- Koonce, S.D.; Brown, J.B. A study of the alcohols of carnauba wax. Oil Soap 1944. [Google Scholar] [CrossRef]
- Deroux, M.; Laguna, A.; Méndez, E.; Cora, M. Chemical study of the Carnauba (Copernicia cerifera Martius) wax. Rev. CENICCiencias Químicas 2003, 34, 85–90. [Google Scholar]
- Dwivedi Atul, P.; Ghosal, G.K.; Belkhode, P.N. IJSRST—Publication Details. Int. J. Sci. Res. Sci. Technol. 2017, 3, 994–997. [Google Scholar]
- Freitas, G.B.; Duncke, A.C.; Barbato, C.N.; de Oliveira, M.C.K.; Pinto, J.C.; Nele, M. Influence of wax chemical structure on W/O emulsion rheology and stability. Colloids Surf. Physicochem. Eng. Asp. 2018, 558, 45–56. [Google Scholar] [CrossRef]
- Ormsby, B.; Cross, M.; Kampasakali, E.; Chantrier Aasen, L.; Smither, P.; Aasen, L.C.; Smithen, P. A preliminary evaluation of artists’ and conservation varnishes for acrylic emulsion paint films. In ICOM-CC 16th Trienn. Conf. Lisbon 19-23 Sept. 2011 Prepr; ICOM: Osaka, Japan, 2011. [Google Scholar]
- Beri, A.; Norton, J.E.; Norton, I.T. Effect of emulsifier type and concentration, aqueous phase volume and wax ratio on physical, material and mechanical properties of water in oil lipsticks. Int. J. Cosmet. Sci. 2013, 35, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Tumosa, C.S.; Mecklenburg, M.F. Weight Changes in Acrylic Emulsion Paints and the Implications for Accelerated Ageing. WAAC Newsl. 2003, 25, 12–14. [Google Scholar]
- The Use of Wax Emulsions in Coatings and Inks. Available online: https://www.pcimag.com/articles/83055-the-use-of-wax-emulsions-in-coatings-and-inks (accessed on 18 August 2020).
- Enhancing Secondary Properties in OEM Wood Coatings. Available online: https://www.azom.com/article.aspx?ArticleID=4906 (accessed on 18 August 2020).
- Lozhechnikova, A.; Bellanger, H.; Michen, B.; Burgert, I.; Österberg, M. Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef] [Green Version]
- Chiantore, O.; Lazzari, M. Photo-oxidative stability of paraloid acrylic protective polymers. Polymer (Guildf.) 2001, 42. [Google Scholar] [CrossRef]
- Palkovská, M.; Slovák, V.; Šubrt, J.; Boháček, J.; Barbieriková, Z.; Brezová, V.; Fajgar, R. Investigation of the thermal decomposition of a new titanium dioxide material: TA/MS and EPR study. J. Therm. Anal. Calorim. 2016, 125, 1071–1078. [Google Scholar] [CrossRef]
- de la Rie, E.R.; Michelin, A.; Ngako, M.; Del Federico, E.; Del Grosso, C. Photo-catalytic degradation of binding media of ultramarine blue containing paint layers: A new perspective on the phenomenon of “ultramarine disease” in paintings. Polym. Degrad. Stab. 2017, 144, 43–52. [Google Scholar] [CrossRef]
- Hu, W.; Huang, J.; Zhang, X.; Zhao, S.; Pei, L.; Zhang, C.; Liu, Y.; Wang, Z. A mechanically robust and reversibly wettable benzoxazine/epoxy/mesoporous TiO2 coating for oil/water separation. Appl. Surf. Sci. 2020, 507, 145168. [Google Scholar] [CrossRef]
- Sanchis, M.R.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface modification of low density polyethylene (LDPE) film by low pressure O2 plasma treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Cao, J.; Mei, C. Improved Hydrophobicity and Dimensional Stability of Wood Treated with Paraffin/Acrylate Compound Emulsion through Response Surface Methodology Optimization. Polymers 2020, 12, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goslinska, M.; Heinrich, S. Characterization of waxes as possible coating material for organic aerogels. Powder Technol. 2019. [Google Scholar] [CrossRef]
- Götz, A.; Nikzad-Langerodi, R.; Staedler, Y.; Bellaire, A.; Saukel, J. Apparent penetration depth in attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy of Allium cepa L. epidermis and cuticle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117460. [Google Scholar] [CrossRef]
- Zaher, N.A.; Melegy, M.S.; Guirguis, O.W. Thermal and Structural Analyses of PMMA/TiO2 Nanoparticles Composites. Nat. Sci. 2014, 6, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Thermal Analysis of Phase Change Materials-Three Organic Waxes using TGA, DSC, and Modulated DSC®; TA Instrument: New Castle, DE, USA, 2016.
- Crespi, M.S.; Hikosaka, M.Y.; Do Amaral, G.C.A.; Ribeiro, C.A. Kinetic parameters obtained for thermal decomposition of acrylic resins present in commercial paint emulsions. J. Therm. Anal. Calorim. 2007, 88, 669–672. [Google Scholar] [CrossRef]
- Whitmore, P.M.; Colaluca, V. Natural and accelerated aging of an artists’ acrylic paint medium. Polym. Prepr. 1992, 204, 320. [Google Scholar]
- Liufu, S.-C.; Xiao, H.-N.; Li, Y.-P. Thermal analysis and degradation mechanism of polyacrylate/ZnO nanocomposites. Polym. Degrad. Stab. 2005. [Google Scholar] [CrossRef]










| Mock-Ups | Description | PVC (%) * | Film Thickness (μm) |
|---|---|---|---|
| PE | Edelwachs | - | 190 ± 10 |
| TW | Edelwachs + Titanum White | 25% | 170 ± 13 |
| ZW | Edelwachs + Zinc White | 32% | 289 ± 44 |
| UB | Edelwachs + Ultramarine Blue | 31% | 220 ± 13 |
| Name | XRF Results * | FT-IR-ATR Results | Comments |
|---|---|---|---|
| TW | Ti +++ | 481 cm−1 TiO2 | Titanium Oxide |
| ZW | Zn +++, Fe (tr) | 500–400 cm−1 oxides | Zinc Oxide |
| UB | S ++, Si +, Al +, Ti +, Fe +, Na (tr), Ca (tr) | 998 cm−1 Si-O-Si 443 cm−1 Si-O-Si | Ultramarine Blue with traces due to natural impurities |
| RT (min) (min) | ** M+ | COMPOUND | RT (min) (min) | ** M+ | COMPOUND |
|---|---|---|---|---|---|
| 0.94 | 56 (41) | 2 butene | 9.34 | 242 (55) | 1-Hexadecanol |
| 1.29 | 72 (44) | Butanal | 10.56 | 244 (115) | Glutaric acid* |
| 1.38 | 86 (55) | Methyl Acrylate | 10.81 | 256 (57) | 1-Hexadecanol, 2-methyl- |
| 1.46 | 88 (29) | Methyl propionate | 10.88 | 239 (127) | Pyrrolidine, 1-(7-oxo-2,4,6-trimethylheptanoyl)- |
| 1.69 | 74 (56) | 1-butanol | 10.92 | 536 (43) | 1-Heptatriacotanol |
| 1.80 | 102 (43) | Propanoic acid, 2-methyl- * | 11.44 | 252 (82) | 13-Heptadecyn-1-ol |
| 1.99 | 100 (41) | Methyl methacrylate | 11.49 | 266 (55) | 9-Nonadecene |
| 2.70 | 112 (43) | 1-octene | 12.14 | 256 (57) | 1-Hexadecanol, 2-methyl- |
| 2.82 | 114 (55) | Butanoic acid, 2-methylene- * | 12.33 | 270 (74) | Hexadecanoic acid * |
| 2.95 | 116 (43) | Acetic acid, butyl ester | 12.61 | 278 (79) | 10-Heptadecen-8-ynoic acid (E)- * |
| 3.05 | 86 (71) | 2-Buten-1-ol, 2-methyl- | 12.76 | 256 (57) | 1-Hexadecanol, 2-methyl- |
| 3.38 | 128 (41) | Butanoic acid, 3-methyl-2-methylene- * | 13.39 | 314 (41) | 4-Methoxycarbonylmethylundec-3-enedioic acid * |
| 3.84 | 128 (55) | n-Butyl Acrylate | 13.43 | 296 (55) | trans-13-Octadecenoic acid * |
| 3.94 | 130 (57) | Propanoic acid * | 13.56 | 314 (41) | 4-Methoxycarbonylmethylundec-3-enedioic acid * |
| 4.25 | 142 (83) | 4-Pentenoic acid, 2,4-dimethyl- * | 13.60 | 298 (74) | Heptadecanoic acid, 16-methyl- * |
| 4.55 | 126 (67) | 4-Pentenoic acid, 2-methylene- * | 14.04 | 472 (57) | Docosyl pentafluoropropionate |
| 4.70 | 142 (41) | n-Butyl methacrylate | 14.15 | 298 (74) | Octadecanoic acid * |
| 4.94 | 148 (49) | 2-Propanol, 1-(2-methoxy-1-methylethoxy)- | 14.71 | 490 (57) | 17-Pentatriacontene |
| 5.05 | 148 (59) | 2-Propanol, 1-(2-methoxypropoxy)- | 14.94 | 326 (74) | Eicosanoic acid * |
| 5.58 | 156 (83) | n-Butyl tiglate | 15.13 | N/A (134) | trimer n-butyl-acrylate |
| 5.84 | 210 (41) | E-11,13-Tetradecadien-1-ol | 16.12 | 366 (43) | Octadecane, 3-ethyl-5-(2-ethylbutyl)- |
| 6.39 | 170 (97) | Cyclopentanecarboxylic acid * | 17.48 | 604 (57) | Tritetracontane |
| 6.42 | 122 (122) | Phenol, 2,5-dimethyl- | 17.69 | 382 (74) | Tetracosanoic acid * |
| 6.80 | 168 (55) | Cyclopentane, 1-methyl-2-(4-methylpentyl)-, trans- | 18.12 | 490 (57) | 17-Pentatriacontene |
| 6.97 | 170 (77) | 2,4-Octadienoic acid, 7-hydroxy-*[R-(E, E)]- | 18.78 | 490 (57) | 17-Pentatriacontene |
| 7.26 | 290 (74) | 13,16-Octadecadiynoic acid * | 18.80 | 618 (57) | Tetratetracontane |
| 7.38 | 186 (81) | 2-Carboxymethyl-3-methyl-cyclopentanecarboxylic acid | 19.01 | 410 (74) | Hexacosanoic acid * |
| 7.69 | 182 (43) | 1-Tridecene | 19.43 | N/A (57) | C32 alcohol, methoxy Carnaubawax |
| 7.90 | 278 (79) | 10-Heptadecen-8-ynoic acid (E)- * | 19.72 | 504 (55) | 9-Hexadecenoic acid, 9-octadecenyl ester, (Z, Z)- |
| 8.09 | 224 (74) | 12-Tridecynoic acid * | 20.07 | 604 (57) | Tritetracontane |
| 8.16 | 154 (95) | 2-Octynoic acid * | 20.28 | 438 (74) | Octacosanoic acid * |
| 8.47 | 294 (41) | 8-Octadecynoic acid * | 20.69 | 612 (57) | Dotriacontyl pentafluoropropionate |
| 8.54 | 242 (55) | 1-Hexadecanol | 21.86 | 490 (57) | 17-Pentatriacontene |
| 8.85 | 174 (59) | Butanedioic acid, 2,3-dimethyl- * | 22.18 | 594 (82) | Tetracontane-1,40-diol |
| 9.04 | 200 (43) | 4-Pentenoic acid, 4-methoxycarbonyl- * | 22.58 | N/A (57) | C32 alcohol, methoxy Carnaubawax |
| 9.28 | 294 (81) | 10-Octadecynoic acid * |
| Mock-Ups | Colour Changes (L*a*b*) | ||||
|---|---|---|---|---|---|
| ΔE | ΔL* | Δa* | Δb* | ||
| PE | PEn.ag | 2.66 | 0.55 | 0.10 | −2.60 |
| PEHT | 36.84 | −15.18 | 12.09 | 31.31 | |
| PEUV | 12.85 | 2.14 | 1.27 | −12.61 | |
| PEHR | 3.63 | 3.34 | 0.79 | 1.20 | |
| ZW | ZWn.ag. | 0.67 | −0.10 | 0.06 | 0.66 |
| ZWHT | 10.62 | −4.79 | 1.22 | 9.39 | |
| ZWUV | 5.05 | 1.18 | 0.30 | −4.90 | |
| ZWHR | 0.13 | 0.03 | −0.09 | −0.10 | |
| TW | TWn.ag. | 0.45 | −0.25 | 0.08 | −0.36 |
| TWHT | 3.57 | −0.90 | 0.24 | 3.45 | |
| TWUV | 1.66 | 0.69 | 0.23 | −1.49 | |
| TWHR | 0.14 | 0.05 | 0.07 | −0.11 | |
| UB | UBn.ag. | 0.77 | −0.53 | 0.17 | 0.53 |
| UBHT | 15.25 | −7.22 | −5.19 | 12.39 | |
| UBUV | 1.58 | 0.97 | −0.01 | −1.25 | |
| UBHR | 1.21 | −0.85 | 0.31 | 0.81 | |
| Mock-Ups | Contact Angles (θ) | |
|---|---|---|
| PE | PE (t0) | 56.16 |
| PEn.ag | 64.15 | |
| PEHT | 88.09 | |
| PEUV | 76.11 | |
| PEHR | 50.79 | |
| ZW | ZW (t0) | 51.09 |
| ZWn.ag. | 67.26 | |
| ZWHT | 83.84 | |
| ZWUV | 78.19 | |
| ZWHR | 62.88 | |
| TW | TW(t0) | 48.94 |
| TWn.ag. | 58.88 | |
| TWHT | 93.67 | |
| TWUV | 74.54 | |
| TWHR | 67.15 | |
| UB | UB (t0) | 51.09 |
| UBn.ag. | 61.85 | |
| UBHT | 86.18 | |
| UBUV | 75.28 | |
| UBHR | 56.91 | |
| Mock-Ups | Ageing Procedures | ||||
|---|---|---|---|---|---|
| Natural Ageing | High Temperatures | UV Irradiation | 85% RH | ||
| Morphological variations | PE | - | - | - | - |
| TW | - | + | - | - | |
| ZW | + | + | + | - | |
| UB | - | - | - | - | |
| Colour variations (DE > 5) | PE | - | + | + | - |
| TW | - | - | - | - | |
| ZW | - | + | + | - | |
| UB | - | + | - | - | |
| Chemical variations (FT-IR-ATR) | PE | - | - | -/+ | - |
| TW | - | +/- | -/+ | - | |
| ZW | - | +/- | - | - | |
| UB | - | - | +/- | - | |
| Wettability (Ɵ) | PE | > | > | > | > |
| TW | > | >> | >> | >> | |
| ZW | > | > | > | > | |
| UB | > | >> | >> | > | |
| Mock-Ups | Total Weight Loss % | Glass Transition (Tg) | |
|---|---|---|---|
| PE | T0 | 99.8 | 36 °C |
| UV | 99.9 | 36 °C | |
| HT | 99.9 | 38 °C | |
| UB | T0 | 37.0 | 30 °C |
| UV | 39.9 | 29 °C | |
| HT | 37.1 | 31 °C | |
| TW | T0 | 49.3 | 36 °C |
| UV | 48.8 | 38 °C | |
| HT | 44.9 | 38 °C | |
| ZW | T0 | 39.1 | 36 °C |
| UV | 31.3 | 38 °C | |
| HT | 37.1 | 36 °C | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, F.C.; Balliana, E.; Perra, E.; Zendri, E. Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art. Polymers 2020, 12, 1925. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091925
Izzo FC, Balliana E, Perra E, Zendri E. Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art. Polymers. 2020; 12(9):1925. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091925
Chicago/Turabian StyleIzzo, Francesca Caterina, Eleonora Balliana, Emanuela Perra, and Elisabetta Zendri. 2020. "Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art" Polymers 12, no. 9: 1925. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12091925