Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
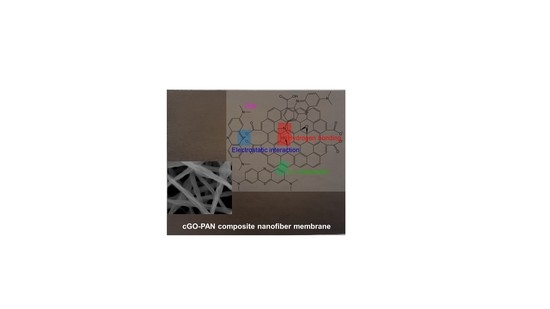
2.2. Preparation of cGO–PAN Composite Nanofibers
2.3. Removal of Dyes Using the Composite Nanofiber Membranes
2.4. Characterization
3. Results and Discussion
3.1. Structural and Compositional Properties of Composite Nanofiber Membranes
3.2. Wettability and Pore Properties of Composite Nanofiber Membranes
3.3. Dye Removal Efficiency of Composite Nanofiber Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Electrospun Nanofibers, 1st ed.; Woodhead Publishing: Cambridge, UK, 2017.
- Agarwal, S.; Burgard, M.; Greiner, A.; Wendorff, J. Electrospinning: A Practical Guide to Nanofibers; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nnanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, B.; Saha, M.C. Investigation on jet stability, fiber diameter, and tensile properties of electrospun polyacrylonitrile nanofibrous yarns. J. Appl. Polym. Sci. 2015, 132, 41918. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Kim, J.-H.; Lee, T.; Byun, H. Electrospun PAN–GO composite nanofibers as water purification membranes. J. Appl. Polym. Sci. 2018, 135, 45858. [Google Scholar] [CrossRef]
- Wang, Q.; Du, Y.; Feng, Q.; Huang, F.; Lu, K.; Liu, J.; Wei, Q. Nanostructures and surface nanomechanical properties of polyacrylonitrile/graphene oxide composite nanofibers by electrospinning. J. Appl. Polym. Sci. 2013, 128, 1152–1157. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Dhakate, S.R. Synthesis and characterization of multiwalled CNT–PAN based composite carbon nanofibers via electrospinning. SpringerPlus 2016, 5, 483. [Google Scholar] [CrossRef] [Green Version]
- Ke, H.; Pang, Z.; Xu, Y.; Chen, X.; Fu, J.; Cai, Y.; Huang, F.; Wei, Q. Graphene oxide improved thermal and mechanical properties of electrospun methyl stearate/polyacrylonitrile form-stable phase change composite nanofibers. J. Therm. Anal. Calorim. 2014, 117, 109–122. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Hosseini Ravandi, S.A.; Sadrjahani, M. Mechanical and structural characterizations of simultaneously aligned and heat treated PAN nanofibers. J. Appl. Polym. Sci. 2012, 124, 3529–3537. [Google Scholar] [CrossRef]
- Hou, X.; Yang, X.; Zhang, L.; Waclawik, E.; Wu, S. Stretching-induced crystallinity and orientation to improve the mechanical properties of electrospun PAN nanocomposites. Mater. Des. 2010, 31, 1726–1730. [Google Scholar] [CrossRef]
- Panapoy, M.; Dankeaw, A.; Ksapabutr, B. Electrical conductivity of PAN-based carbon nanofibers prepared by electrospinning method. Thammasat Int. J. Sci. Tech. 2008, 13, 11–17. [Google Scholar]
- Kausar, A. Polyacrylonitrile-based nanocomposite fibers: A review of current developments. J. Plast. Film Sheeting 2019, 35, 295–316. [Google Scholar] [CrossRef]
- Uddin, M.E.; Layek, R.K.; Kim, N.H.; Hui, D.; Lee, J.H. Preparation and properties of reduced graphene oxide/polyacrylonitrile nanocomposites using polyvinyl phenol. Compos. Part B 2015, 80, 238–245. [Google Scholar] [CrossRef]
- Suriyaraj, S.P.; Begam, M.B.; Deepika, S.G.; Biji, P.; Selvakumar, R. Photocatalytic removal of nitrate using TiO2/PAN nanofiber membrane synthesized by co-electrospinning process. Water Sci. Technol. Water Supply 2014, 14, 554–560. [Google Scholar] [CrossRef]
- Huang, X. A facile approach to make high performance nano-fiber reinforced composite separator for lithium ion batteries. J. Power Sources 2016, 323, 17–22. [Google Scholar] [CrossRef]
- Pant, H.R.; Park, C.H.; Tijing, L.D.; Amarjargal, A.; Lee, D.-H.; Kim, C.-S. Bimodal fiber diameter distributed graphene oxide/nylon-6 composite nanofibrous mats via electrospinning. Colloids Surf. A 2012, 407, 121–125. [Google Scholar] [CrossRef]
- Naseeb, N.; Mohammed, A.A.; Laoui, T.; Khan, Z. A novel PAN-GO-SiO2 hybrid membrane for separating oil and water from emulsified mixture. Materials 2019, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Park, Y.; Park, C.; Seo, Y.; Kim, J.-H.; Hou, J.; Byun, H. Regulating the integrity of diverse composite nanofiber membranes using an organoclay. J. Membr. Sci. 2020, 598, 117670. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.; Hwang, I.-C.; Kim, K. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef]
- Liang, B.; Zhan, W.; Qi, G.; Lin, S.; Nan, Q.; Liu, Y.; Cao, B.; Pan, K. High performance graphene oxide/polyacrylonitrile composite pervaporation membranes for desalination applications. J. Mater. Chem. A 2015, 3, 5140–5147. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, X.; Xue, Q.; He, D.; Guo, Q. Antifouling hydrolyzed polyacrylonitrile/graphene oxide membrane with spindle-knotted structure for highly effective separation of oil-water emulsion. J. Membr. Sci. 2017, 532, 38–46. [Google Scholar] [CrossRef]
- Gupta, S.; Narayan, J. Direct conversion of Teflon into nanodiamond films. Mater. Res. Lett. 2020, 8, 408–416. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Abuzade, R.A.; Zadhoush, A.; Gharehaghaji, A.A. Air permeability of electrospun polyacrylonitrile nanoweb. J. Appl. Polym. Sci. 2012, 126, 232–243. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.-H.; Yu, X.-L.; Kang, F. Graphene oxide-embedded porous carbon nanofiber webs by electrospinning for capacitive deionization. Colloids Surf. A 2014, 444, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Das, S.; Wajid, A.S.; Bhattacharia, S.K.; Wilting, M.D.; Rivero, I.V.; Green, M.J. Electrospinning of polymer nanofibers loaded with noncovalently functionalized graphene. J. Appl. Polym. Sci. 2013, 128, 4040–4046. [Google Scholar] [CrossRef]
- Lei, S.; Zhong, S.; Wang, Y.; Tong, Y.; Xu, L. Preparation of monodisperse reduced graphene oxide/polyacrylonitrile composite and its thermal-induced structural transformation. Mater. Lett. 2015, 161, 108–111. [Google Scholar] [CrossRef]
- Mehrpouya, F.; Foroughi, J.; Naficy, S.; Razal, J.M.; Naebe, M. Nanostructured electrospun hybrid graphene/polyacrylonitrile yarns. Nanomaterials 2017, 7, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zabihi, O.; Wang, J.; Li, Q.; Wang, J.; Lei, W.; Naebe, M. Hydrophilic PAN based carbon nanofibres with improved graphitic structure and enhanced mechanical performance using ethylenediamine functionalized graphene. RSC Adv. 2017, 7, 2621–2628. [Google Scholar] [CrossRef] [Green Version]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Q.; Zhang, T. Preparation of cationic surfactant intercalated graphene oxide and quantitative determination of the interlamellar spacing. Fuller. Nanotub. Carbon Nanostruct. 2014, 23, 196–202. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, Z.; Liu, D.; He, X. Microwave-assisted modification of activated carbon with cationic surfactants for enhancement of naphthalene adsorption. Korean J. Chem. Eng. 2018, 35, 557–566. [Google Scholar] [CrossRef]
- Sasaki, K.-I.; Tokura, Y.; Sogawa, T. The origin of Raman D band: Bonding and antibonding orbitals in graphene. Crystals 2013, 3, 120–140. [Google Scholar] [CrossRef] [Green Version]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Perumbilavil, S.; Sankar, P.; Rose, T.P.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–264. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Narayan, J. Reduced graphene oxide/amorphous carbon P−N junctions: Nanosecond laser patterning. ACS Appl. Mater. Interfaces 2019, 11, 24318–24330. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.P.; Kesavan, T.; Kalita, G.; Ragupathy, P.; Narayanan, T.N.; Pattanayak, D.K. On the large capacitance of nitrogen doped graphene derived by a facile route. RSC Adv. 2014, 4, 38689–38697. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, A.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef]
- Swaminathan, S.; Muthumanickkam, A.; Imayathamizhan, N.M. An effective removal of methylene blue dye using polyacrylonitrile yarn waste/graphene oxide nanofibrous composite. Int. J. Environ. Sci. Technol. 2015, 12, 3499–3508. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, Z.; Karasavvidis, C.; Dimirkou, A.; Antoniadis, V. Adsorption of methylene blue and methyl red dyes from aqueous solutions onto modified zeolites. Water Sci. Technol. 2013, 67, 1129–1136. [Google Scholar] [CrossRef]
- Borhani, S.; Asadi, A.; Dabbagh, H.A. Preparation and characterization of PAN nanofibers containing boehmite nanoparticles for the removal of microbial contaminants and cadmium ions from water. J. Water Health 2020, 18, 106–117. [Google Scholar] [CrossRef]
- Hashim, N.; Muda, Z.; Hussein, M.Z.; Isa, I.M.; Mohamed, A.; Kamari, A.; Bakar, S.A.; Mamat, M.; Jaafar, A. A brief review on recent graphene oxide-based material nanocomposites: Synthesis and applications. J. Mater. Environ. Sci. 2016, 7, 3225–3243. [Google Scholar]
- Martin, D.F.; Alessio, R.J.; McCane, C.H. Removal of synthetic food dyes in aqueous solution by Octolig®. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2013, 48, 495–500. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Z.; Gou, J.; Dong, S. Highly efficient adsorption and removal of Chrysoidine Y from aqueous solution by magnetic graphene oxide nanocomposite. Arab. J. Chem. 2019, 12, 3064–3074. [Google Scholar] [CrossRef] [Green Version]
- Hassaninejad-Darzi, S.K.; Torkamanzadeh, M. Simultaneous UV-Vis spectrophotometric quantification of ternary basic dye mixtures by partial least squares and artificial neural networks. Water Sci. Technol. 2016, 74, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Minor, E.C.; Austin, J.A.; Sun, L.; Gauer, L.; Zimmerman, R.C.; Mopper, K. Mixing effects on light exposure in a large-lake epilimnion: A preliminary dual-dye study. Limnol. Oceanogr. Methods 2016, 14, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.T. Adsorbents: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Mondal, N.K.; Bhaumik, R.; Roy, P. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int. J. Environ. Sci. Technol. 2014, 11, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef] [Green Version]
- Igwe, J.C.; Mbonu, O.F.; Abia, A.A. Sorption kinetics, intraparticle diffusion and equilibrium partitioning of azo dyes on great millet (Andropogon sorghum) waste biomass. J. Appl. Sci. 2007, 7, 2840–2847. [Google Scholar]
- Fil, B.A.; Yilmaz, M.T.; Bayar, S.; Elkoca, M.T. Investigation of adsorption of the dyestuff Astrazon Red Violet 3RN (basic violet 16) on montmorillonite clay. Braz. J. Chem. Eng. 2014, 31, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Suteu, D.; Malutan, T. Industrial cellolignin wastes as adsorbent for removal of methylene blue dye from aqueous solutions. BioResources 2013, 8, 427–446. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Liu, L.; Zhang, Y.; Liao, Q.; Yu, Q.; Meng, R.; Yao, J. Efficient removal of cationic and anionic dyes from aqueous solution using cellulose-g-p(AA-co-AM) bio-adsorbent. BioResources 2017, 12, 3413–3424. [Google Scholar] [CrossRef] [Green Version]
- Do Nascimento, G.E.; Duarte, M.M.M.B.; Campos, N.F.; De Menezes Barbosa, C.M.; Da Silva, V.L. Adsorption of the reactive gray BF-2R dye on orange peel: Kinetics and equilibrium studies. Desalin. Water Treat. 2013, 52, 1578–1588. [Google Scholar] [CrossRef]







| Sample Name | Bubble Point (nm) | Average Pore Size (nm) | Smallest Pore Size (nm) | Thickness (µm) |
|---|---|---|---|---|
| Bare PAN | 507.5 | 306.0 | 283.7 | 90–94 |
| cGO02 PAN | 452.7 | 302.2 | 244.3 | 91–99 |
| cGO04 PAN | 460.4 | 319.0 | 290.2 | 99–104 |
| cGO06 PAN | 494.7 | 325.8 | 319.5 | 93–99 |
| cGO10 PAN | 521.6 | 354.9 | 306.2 | 87–93 |
| cGO20 PAN | 882.3 | 253.3 | 222.1 | 93–95 |
| cGO30 PAN | 966.7 | 258.3 | 208.5 | 102–104 |
| Kinetic Model and Parameters | First-Order | Second-Order | Diffusion | Elovich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min) | R2 | qe (mg/g) | k2 (min) | R2 | k3 | R2 | ae (mg/g·min) | be (g/mg) | R2 | |
| MR | 2.773 | 0.0106 | 97.9 | 4.444 | 0.198 | 88.1 | 0.245 | 69.4 | 0.0899 | 1.109 | 99.6 |
| MB | 2.389 | 0.0038 | 98.8 | 5.236 | 3.442 | 30.0 | 0.111 | 99.6 | 0.0283 | 2.481 | 99.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Yun, J.; Seo, Y.; Byun, H.; Hou, J.; Kim, J.-H. Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes. Polymers 2020, 12, 2009. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092009
Jang W, Yun J, Seo Y, Byun H, Hou J, Kim J-H. Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes. Polymers. 2020; 12(9):2009. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092009
Chicago/Turabian StyleJang, Wongi, Jaehan Yun, Younggee Seo, Hongsik Byun, Jian Hou, and Jun-Hyun Kim. 2020. "Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes" Polymers 12, no. 9: 2009. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092009