A Kirigami Approach of Patterning Membrane Actuators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electropolymerization and Pattern Design
2.3. Fabrication and Actuation
2.4. Characterizations
3. Results and Discussion
3.1. Device Design
3.2. Characterization of Membrane Actuators
3.3. Displacement of Membrane Actuators
3.3.1. Simulation of Bending Displacements
3.3.2. The Improvement of Displacement by Patterning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, H.; Jung, S.; Jeong, S.; Kim, G.; Lee, K. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 2015, 6, 6503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Moliton, A.; Hiorns, R.C. Review of electronic and optical properties of semiconducting π-conjugated polymers: Applications in optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Harjo, M.; Järvekülg, M.; Tamm, T.; Otero, T.F.; Kiefer, R. Concept of an artificial muscle design on polypyrrole nanofiber scaffolds. PLoS ONE 2020, 15, e0232851. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Kiefer, R.; Elhi, F.; Anbarjafari, G.; Martinez, J.G.; Tamm, T. A biomimetic approach to increasing soft actuator performance by friction reduction. Polymers 2020, 12, 1120. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Baraban, M.; Wong, J.; Matsudaira, P.; Van Oudenaarden, A.; Mahadevan, L. Power-limited contraction dynamics of Vorticella convallaria: An ultrafast biological spring. Biophys. J. 2008, 94, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Carpi, F.; Migliore, A.; Serra, G.; De Rossi, D. Helical dielectric elastomer actuators. Smart Mater. Struct. 2005, 14, 1210–1216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Qiu, C.; Dai, J.S. Helical kirigami-enabled centimeter-scale worm robot with shape-memory-alloy linear actuators. J. Mech. Robot. 2015, 7, 021014. [Google Scholar] [CrossRef]
- Tadesse, Y.; Grange, R.W.; Priya, S. Synthesis and cyclic force characterization of helical polypyrrole actuators for artificial facial muscles. Smart Mater. Struct. 2009, 18, 085008. [Google Scholar] [CrossRef]
- Madden, J.D.; Lafontaine, S.R.; Hunter, I.W. Fabrication by Electrodeposition: Building 3D Structures and Polymer Actuators. In Proceedings of the Sixth International Symposium on Micro Machine and Human Science, Nagoya, Japan, 4–6 October 1995; pp. 77–81. [Google Scholar]
- Aziz, S.; Martinez, J.G.; Foroughi, J.; Spinks, G.M.; Jager, E.W.H. Artificial Muscles from Hybrid Carbon Nanotube-Polypyrrole-Coated Twisted and Coiled Yarns. Macromol. Mater. Eng. 2020, 305, 2000421. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Christophersen, M.; Shapiro, B.; Smela, E. Characterization and modeling of PPy bilayer microactuators. Part 1. Curvature. Sens. Actuators B Chem. 2006, 115, 596–609. [Google Scholar] [CrossRef]
- Zainudeen, U.L.; Careem, M.A.; Skaarup, S. PEDOT and PPy conducting polymer bilayer and trilayer actuators. Sens. Actuators B Chem. 2008, 134, 467–470. [Google Scholar] [CrossRef]
- Wang, H.; Tjahyono, S.S.; MacDonald, B.; Kilmartin, P.A.; Travas-Sejdic, J.; Kiefer, R. Robotic Fish Based on a Polymer Actuator. In Proceedings of the 2007 Australasian Conference on Robotics and Automation ACRA 2007, Brisbane, Australia, 10–12 December 2007. [Google Scholar]
- Alici, G.; Gunderson, D. A Bio-inspired Robotic Locomotion System Based on Conducting Polymer Actuators. In Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics; IEEE: Singapore, 2009; pp. 998–1004. [Google Scholar]
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, 1808210. [Google Scholar] [CrossRef]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, e1600327. [Google Scholar] [CrossRef] [Green Version]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuation. Sens. Actuators B Chem. 2016, 233, 461–469. [Google Scholar] [CrossRef]
- Khaldi, A.; Falk, D.; Bengtsson, K.; Maziz, A.; Filippini, D.; Robinson, N.D.; Jager, E.W.H. Patterning Highly Conducting Conjugated Polymer Electrodes for Soft and Flexible Microelectrochemical Devices. ACS Appl. Mater. Interfaces 2018, 10, 14978–14985. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lau, K.T.; Shepherd, R.; Wu, Y.; Wallace, G.; Diamond, D. Performance characteristics of a polypyrrole modified polydimethylsiloxane (PDMS) membrane based microfluidic pump. Sens. Actuators A Phys. 2008, 148, 239–244. [Google Scholar] [CrossRef]
- Sareh, S.; Rossiter, J. Kirigami artificial muscles with complex biologically inspired morphologies. Smart Mater. Struct. 2013, 22, 014004. [Google Scholar] [CrossRef]
- Temmer, R.; Must, I.; Kaasik, F.; Aabloo, A.; Tamm, T. Combined chemical and electrochemical synthesis methods for metal-free polypyrrole actuators. Sens. Actuators B Chem. 2012, 166–167, 411–418. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Gade, V.K.; Shirale, D.J.; Gaikwad, P.D.; Kakde, P.; Savale, P.A.; Kharat, H.J. Synthesis and Characterization of Ppy-PVS, Ppy-pTS, and Ppy-DBS Composite Films. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 107–114. [Google Scholar] [CrossRef]
- Khadka, R.; Aydemir, N.; Kesküla, A.; Tamm, T.; Travas-Sejdic, J.; Kiefer, R. Enhancement of polypyrrole linear actuation with poly(ethylene oxide). Synth. Met. 2017, 232, 1–7. [Google Scholar] [CrossRef]
- Akin, J.E. Finite Element Analysis Concepts: Via Solidworks; World Scientific Publishing Company: Singapore, 2010; ISBN-13 978-981-4313-01-8. [Google Scholar]
- Timoshenko, S.; Woinowsky-Krieger, S. Theory of Plates and Shells, 2nd ed.; McGraw-Hill-Book Company: New York, NY, USA, 1959; ISBN 0-07-064779-8. [Google Scholar]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 96484–96489. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the electrolyte concentration and substrate on conducting polymer actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Mei, D.; Sun, J.Y.; Zhao, Z.H.; He, X.T. A closed-form solution for the boundary value problem of gas pressurized circular membranes in contact with frictionless rigid plates. Mathematics 2020, 8, 1017. [Google Scholar] [CrossRef]








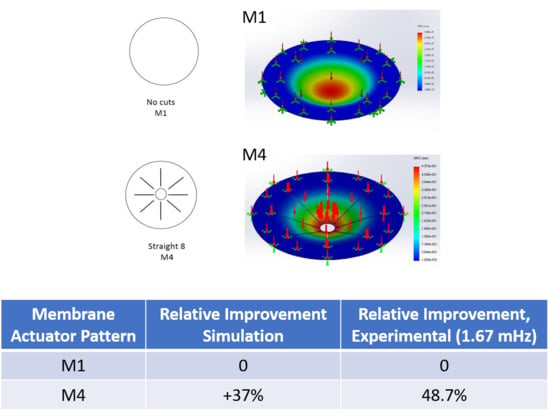
| Membrane Actuator Pattern | Relative Improvement, Simulation | Relative Improvement, Experimental (1.67 mHz) | Relative Improvement, Experimental (16.7 mHz) |
|---|---|---|---|
| M1 | 0 | 0 | 0 |
| M2 | +11.6% | +10% | +11% |
| M3 | +23.7% | +24.4% | +33.1% |
| M4 | +37% | +48.7% | +56.2% |
| M5 | +36% | +36% | +41.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiveste, H.; Kiefer, R.; Haamer, R.E.; Anbarjafari, G.; Tamm, T. A Kirigami Approach of Patterning Membrane Actuators. Polymers 2021, 13, 125. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010125
Kiveste H, Kiefer R, Haamer RE, Anbarjafari G, Tamm T. A Kirigami Approach of Patterning Membrane Actuators. Polymers. 2021; 13(1):125. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010125
Chicago/Turabian StyleKiveste, Harti, Rudolf Kiefer, Rain Eric Haamer, Gholamreza Anbarjafari, and Tarmo Tamm. 2021. "A Kirigami Approach of Patterning Membrane Actuators" Polymers 13, no. 1: 125. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010125