Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strain and Culture Medium
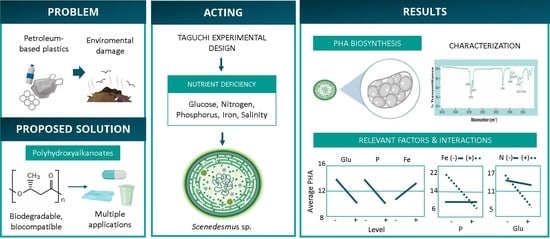
2.2. Experimental Design
2.3. Culture Conditions
2.4. Analytic Methods
2.4.1. Biomass
2.4.2. COD
2.4.3. VFAs
2.4.4. Total Lipid Content
2.4.5. Total Carbohydrate Content
2.5. PHA Extraction and Quantification
3. Results
3.1. Growth and Characterization of Biomass
3.2. Analysis of Bioplastic Production
3.3. Experiment Design and Evaluation of the Relevant Factors
3.4. Influence of Relevant Factors on the Bioproduction of PHA
3.5. Accumulation of Lipids and Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A













References
- Heller, M.C.; Mazor, M.H.; Keoleian, G.A. Plastics in the US: Toward a material flow characterization of production, markets and end of life. Environ. Res. Lett. 2020, 15, 094034. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Dawes, E.A.; Senior, P.J. The Role and Regulation of Energy Reserve Polymers in Micro-organisms. Adv. Microb. Physiol. 1973, 10, 135–266. [Google Scholar] [CrossRef]
- Raza, Z.A.; Riaz, S.; Banat, I.M. Polyhydroxyalkanoates: Properties and chemical modification approaches for their functionalization. Biotechnol. Prog. 2018, 34, 29–41. [Google Scholar] [CrossRef]
- Nitkiewicz, T.; Wojnarowska, M.; Sołtysik, M.; Kaczmarski, A.; Witko, T.; Ingrao, C.; Guzik, M. How sustainable are biopolymers? Findings from a life cycle assessment of polyhydroxyalkanoate production from rapeseed-oil derivatives. Sci. Total Environ. 2020, 749, 141279. [Google Scholar] [CrossRef]
- Kalia, V.C.; Ray, S.; Patel, S.K.S.; Singh, M.; Singh, G.P. The Dawn of Novel Biotechnological Applications of Polyhydroxyalkanoates. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 1–11. [Google Scholar]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.-Q.; Zhang, J. Microbial polyhydroxyalkanoates as medical implant biomaterials. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Zavala-Yoe, R.; Bilal, M.; Ramirez-Mendoza, R.A.; Parra-Saldivar, R.; Iqbal, H.M.N. Poly-3-hydroxybutyrate-based constructs with novel characteristics for drug delivery and tissue engineering applications—A review. Polym. Eng. Sci. 2020, 60, 1760–1772. [Google Scholar] [CrossRef]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.; Abdal-Hay, A.; Skouras, A.; Tiboni, M.; Casettari, L.; Guarino, V. Polyhydroxyalkanoate (PHA): Applications in drug delivery and tissue engineering. Expert Rev. Med. Devices 2019, 16, 467–482. [Google Scholar] [CrossRef]
- Li, Z.; Lim, J. Biodegradable polyhydroxyalkanoates nanocarriers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 607–634. [Google Scholar]
- Michalak, M.; Kurcok, P.; Hakkarainen, M. Polyhydroxyalkanoate-based drug delivery systems. Polym. Int. 2017, 66, 617–622. [Google Scholar] [CrossRef]
- Bonnenfant, C.; Gontard, N.; Aouf, C. Active packaging: Incorporation of polyphenols in polyhydroxyalkanoate (PHA): Thermal stabilization and antioxidant properties. In Proceedings of the Biopolymers and Sustainable Composites, Valencia, Spain, 4–5 March 2020. [Google Scholar]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Masood, F. Polyhydroxyalkanoates in the Food Packaging Industry. In Nanotechnology Applications in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 153–177. [Google Scholar]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Alday, P.; Ibrahim, O.A.; Fernández, B.; Kjeang, E.; Sabaté, N. A Metal-Free and Biotically Degradable Battery for Portable Single-Use Applications. Adv. Energy Mater. 2017, 7, 1700275. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Arauz, A.O.; Aguilar-Rabiela, A.E.; Vargas-Torres, A.; Rodríguez-Hernández, A.-I.; Chavarría-Hernández, N.; Vergara-Porras, B.; López-Cuellar, M.R. Production and characterization of biodegradable films of a novel polyhydroxyalkanoate (PHA) synthesized from peanut oil. Food Packag. Shelf Life 2019, 20, 100297. [Google Scholar] [CrossRef]
- Umesh, M.; Priyanka, K.; Thazeem, B.; Preethi, K. Biogenic PHA nanoparticle synthesis and characterization from Bacillus subtilis NCDC0671 using orange peel medium. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 996–1004. [Google Scholar] [CrossRef]
- Relinque, J.; de León, A.; Hernández-Saz, J.; García-Romero, M.; Navas-Martos, F.; Morales-Cid, G.; Molina, S. Development of Surface-Coated Polylactic Acid/Polyhydroxyalkanoate (PLA/PHA) Nanocomposites. Polymers 2019, 11, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Fazal, T.; Mushtaq, A.; Rehman, F.; Ullah Khan, A.; Rashid, N.; Farooq, W.; Rehman, M.S.U.; Xu, J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018, 82, 3107–3126. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Song, M.; Pei, H.; Hu, W.; Zhang, S.; Ma, G.; Han, L.; Ji, Y. Identification and characterization of a freshwater microalga Scenedesmus SDEC-8 for nutrient removal and biodiesel production. Bioresour. Technol. 2014, 162, 129–135. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Eloka-Eboka, A.C.; Inambao, F.L. Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl. Energy 2017, 195, 1100–1111. [Google Scholar] [CrossRef]
- Van der Ha, D.; Nachtergaele, L.; Kerckhof, F.-M.; Rameiyanti, D.; Bossier, P.; Verstraete, W.; Boon, N. Conversion of Biogas to Bioproducts by Algae and Methane Oxidizing Bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y. Macro assessment of microalgae-based CO2 sequestration: Environmental and energy effects. Algal Res. 2020, 51, 102066. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, G.; Cao, X.; Wei, D. Molecular characterization of CO2 sequestration and assimilation in microalgae and its biotechnological applications. Bioresour. Technol. 2017, 244, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R.; Garcia-Perez, J.S.; Contreras-Angulo, J.R.; Markou, G.; Muylaert, K.; Rittmann, B.E.; Parra-Saldivar, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Passero, M.; Cragin, B.; Coats, E.R.; McDonald, A.G.; Feris, K. Dairy Wastewaters for Algae Cultivation, Polyhydroxyalkanote Reactor Effluent Versus Anaerobic Digester Effluent. BioEnergy Res. 2015, 8, 1647–1660. [Google Scholar] [CrossRef] [Green Version]
- Arias, D.M.; García, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. New Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef] [PubMed]
- Tharani, D.; Ananthasubramanian, M. Microalgae as Sustainable Producers of Bioplastic. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 373–396. [Google Scholar]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef]
- Miyake, M.; Takase, K.; Narato, M.; Khatipov, E.; Schnackenberg, J.; Shirai, M.; Kurane, R.; Asada, Y. Polyhydroxybutyrate Production from Carbon Dioxide by Cyanobacteria. Appl. Biochem. Biotechnol. 2000, 84–86, 991–1002. [Google Scholar] [CrossRef]
- Ciebiada, M.; Kubiak, K.; Daroch, M. Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms. Int. J. Mol. Sci. 2020, 21, 7204. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.; Srivastava, S. Novel quantitative insights into carbon sources for synthesis of poly hydroxybutyrate in Synechocystis PCC 6803. Photosynth. Res. 2018, 136, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Doello, S.; Gutekunst, K.; Forchhammer, K. PHB is Produced from Glycogen Turn-over during Nitrogen Starvation in Synechocystis sp. PCC 6803. Int. J. Mol. Sci. 2019, 20, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monshupanee, T.; Incharoensakdi, A. Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 2014, 116, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Sharma, L.; Mallick, N.; Mala, J. Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J. Appl. Phycol. 2017, 29, 1213–1232. [Google Scholar] [CrossRef]
- Kaewbai-ngam, A.; Incharoensakdi, A.; Monshupanee, T. Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 2016, 212, 342–347. [Google Scholar] [CrossRef]
- Mendhulkar, V.D.; Shetye, L.A. Synthesis of Biodegradable Polymer Polyhydroxyalkanoate (PHA) in Cyanobacteria Synechococcus elongates Under Mixotrophic Nitrogen- and Phosphate-Mediated Stress Conditions. Ind. Biotechnol. 2017, 13, 85–93. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef] [Green Version]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Passanha, P.; Esteves, S.R.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J. Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour. Technol. 2013, 147, 345–352. [Google Scholar] [CrossRef]
- Fu, F.; Bell, P. Growth, N2 fixation and photosynthesis in a cyanobacterium, Trichodesmium sp., under Fe stress. Biotechnol. Lett. 2003, 25, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol. 1990, 116, 1–18. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Ma, N.; Zhang, Q.; Qu, D.; Li, M. Overvalued allelopathy and overlooked effects of humic acid-like substances on Microcystis aeruginosa and Scenedesmus obliquus competition. Harmful Algae 2018, 78, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Abd El Baky, H.H.; El-Baroty, G.S.; Bouaid, A.; Martinez, M.; Aracil, J. Enhancement of lipid accumulation in Scenedesmus obliquus by Optimizing CO2 and Fe3+ levels for biodiesel production. Bioresour. Technol. 2012, 119, 429–432. [Google Scholar] [CrossRef]
- Das, P.; Ibrahim Thaher, M.; Abdul Quadir Mohd Abdul Hakim, M.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. Optimization of iron dosage for microalgal biomass production as a feedstock for biofuel. Biofuels 2019, 1–9. [Google Scholar] [CrossRef]
- Browning, T.J.; Achterberg, E.P.; Yong, J.C.; Rapp, I.; Utermann, C.; Engel, A.; Moore, C.M. Iron limitation of microbial phosphorus acquisition in the tropical North Atlantic. Nat. Commun. 2017, 8, 15465. [Google Scholar] [CrossRef] [Green Version]
- Obruca, S.; Sedlacek, P.; Koller, M.; Kucera, D.; Pernicova, I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol. Adv. 2018, 36, 856–870. [Google Scholar] [CrossRef]
- An, S.S.; Friedl, T.; Hegewald, E. Phylogenetic Relationships of Scenedesmus and Scenedesmus-like Coccoid Green Algae as Inferred from ITS-2 rDNA Sequence Comparisons. Plant Biol. 1999, 1, 418–428. [Google Scholar] [CrossRef]
- Coesel, P.F.M.; Krienitz, L. Diversity and geographic distribution of desmids and other coccoid green algae. In Protist Diversity and Geographical Distribution; Springer: Berlin, Germany, 2007; pp. 147–158. [Google Scholar]
- Lewis, L.A.; Flechtner, V.R. Cryptic Species of Scenedesmus (Chlorophyta) from Desert Soil Communities of Western North America. J. Phycol. 2004, 40, 1127–1137. [Google Scholar] [CrossRef]
- Chen, S.; He, H.; Zong, R.; Liu, K.; Miao, Y.; Yan, M.; Xu, L. Geographical Patterns of Algal Communities Associated with Different Urban Lakes in China. Int. J. Environ. Res. Public Health 2020, 17, 1009. [Google Scholar] [CrossRef] [Green Version]
- Ruangsomboon, S.; Ganmanee, M.; Choochote, S. Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J. Appl. Phycol. 2013, 25, 867–874. [Google Scholar] [CrossRef]
- Sudesh, K.; Taguchi, K.; Doi, Y. Effect of increased PHA synthase activity on polyhydroxyalkanoates biosynthesis in Synechocystis sp. PCC6803. Int. J. Biol. Macromol. 2002, 30, 97–104. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Chen, J.C.; Kirby, E.D. Surface roughness optimization in an end-milling operation using the Taguchi design method. J. Mater. Process. Technol. 2007, 184, 233–239. [Google Scholar] [CrossRef]
- Wu, G.F.; Shen, Z.Y.; Wu, Q.Y. Modification of carbon partitioning to enhance PHB production in Synechocystis sp. PCC6803. Enzym. Microb. Technol. 2002, 30, 710–715. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Q.; Shen, Z. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Savenkova, L.; Gercberga, Z.; Kizhlo, Z.; Stegantseva, E. Effect of phosphate supply and aeration on poly-β-hydroxybutyrate production in Azotobacter chroococcum. Process Biochem. 1999, 34, 109–114. [Google Scholar] [CrossRef]
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 2000, 6, 87–102. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef]
- Cavonius, L.; Fink, H.; Kiskis, J.; Albers, E.; Undeland, I.; Enejder, A. Imaging of Lipids in Microalgae with Coherent Anti-Stokes Raman Scattering Microscopy. Plant Physiol. 2015, 167, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 2000, 49, 1–14. [Google Scholar] [CrossRef]
- Mallick, N.; Gupta, S.; Panda, B.; Sen, R. Process optimization for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production by Nostoc muscorum. Biochem. Eng. J. 2007, 37, 125–130. [Google Scholar] [CrossRef]
- Vanessa, C.C.; da Cleber, K.S.; Ana, L.T.; Jorge, A.V.C.; Morais, G.M. Polyhydroxybutyrate production by Spirulina sp. LEB 18 grown under different nutrient concentrations. Afr. J. Microbiol. Res. 2015, 9, 1586–1594. [Google Scholar] [CrossRef] [Green Version]
- Kavitha, G.; Kurinjimalar, C.; Sivakumar, K.; Kaarthik, M.; Aravind, R.; Palani, P.; Rengasamy, R. Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int. J. Biol. Macromol. 2016, 93, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Nakai, K.; Miyake, M.; Asada, Y.; Taya, M. Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol. Lett. 2001, 23, 1095–1099. [Google Scholar] [CrossRef]
- Panda, B.; Mallick, N. Enhanced poly-?-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 2007, 44, 194–198. [Google Scholar] [CrossRef]
- Sharma, L.; Mallick, N. Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: Regulation by pH, light–dark cycles, N and P status and carbon sources. Bioresour. Technol. 2005, 96, 1304–1310. [Google Scholar] [CrossRef]
- Bottomley, P.J.; Stewart, W.D.P. ATP pools and transients in the blue-green alga, Anabaena cylindrica. Arch. Microbiol. 1976, 108, 249–258. [Google Scholar] [CrossRef]
- Kessler, B.; Witholt, B. Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J. Biotechnol. 2001, 86, 97–104. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Lee, K. Effects of iron sources on the growth and lipid/carbohydrate production of marine microalga Dunaliella tertiolecta. Biotechnol. Bioprocess Eng. 2017, 22, 68–75. [Google Scholar] [CrossRef]
- Rueler, J.G.; Ades, D.R. The role of iron nutrition in photosynthesis and nitrogen assimilation in SCENEDESMUS QUADRICAUDA (Chlorophyceae). J. Phycol. 1987, 23, 452–457. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Sarath, G.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Prathima Devi, M.; Swamy, Y.V.; Venkata Mohan, S. Nutritional mode influences lipid accumulation in microalgae with the function of carbon sequestration and nutrient supplementation. Bioresour. Technol. 2013, 142, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.I.; Stevens, S.E.J.; Balkwill, D.L. Accumulation of poly-beta-hydroxybutyrate in Spirulina platensis. J. Bacteriol. 1982, 149, 361–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Nigam, S.; Rai, M.P.; Sharma, R. Effect of Nitrogen on Growth and Lipid Content of Chlorella pyrenoidosa. Am. J. Biochem. Biotechnol. 2011, 7, 124–129. [Google Scholar] [CrossRef]
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Deviprasad, K.; Chauhan, V.S.; Ravishankar, G.A.; Sarada, R. Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. Bioresour. Technol. 2013, 144, 28–37. [Google Scholar] [CrossRef]
- Macagnan, K.L.; Alves, M.I.; da Silveira Moreira, A. Approaches for Enhancing Extraction of Bacterial Polyhydroxyalkanoates for Industrial Applications. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 389–408. [Google Scholar]
- Aramvash, A.; Moazzeni Zavareh, F.; Gholami Banadkuki, N. Comparison of different solvents for extraction of polyhydroxybutyrate from Cupriavidus necator. Eng. Life Sci. 2018, 18, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Aramvash, A.; Gholami-Banadkuki, N.; Seyedkarimi, M.-S. An efficient method for the application of PHA-poor solvents to extract polyhydroxybutyrate from Cupriavidus necator. Biotechnol. Prog. 2016, 32, 1480–1486. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Bux, F. Exploration of Microalgae Biorefinery by Optimizing Sequential Extraction of Major Metabolites from Scenedesmus obliquus. Ind. Eng. Chem. Res. 2017, 56, 3407–3412. [Google Scholar] [CrossRef]






| Factors | Levels | ||
|---|---|---|---|
| Variable | Name | Low (1) | High (2) |
| Glucose (g L−1) | A | 1 | 4 |
| Nitrogen (mM) | B | 0 | 17.6 |
| Phosphorus (mM) | C | 0 | 0.23 |
| Iron (Mm) | D | 0 | 0.21 |
| Salinity (g L−1) | E | 0.5 | 2 |
| Run | Glucose (g L−1) | Nitrogen (mM) | Phosphorus (mM) | Iron (mM) | Salinity (g L−1) | Polyhydroxyalkanoate (PHA) (% w/w) | PHA (g L−1) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 0.5 | 9.793 | 0.064 |
| 2 | 1 | 0 | 0 | 0.021 | 2 | 26.25 | 0.171 |
| 3 | 1 | 0 | 0.23 | 0 | 0.5 | 11.68 | 0.082 |
| 4 | 1 | 0 | 0.23 | 0.021 | 2 | 9.075 | 0.073 |
| 5 | 1 | 17.6 | 0 | 0 | 2 | 13.80 | 0.104 |
| 6 | 1 | 17.6 | 0 | 0.021 | 0.5 | 29.92 | 0.239 |
| 7 | 1 | 17.6 | 0.23 | 0 | 2 | 12.20 | 0.085 |
| 8 | 1 | 17.6 | 0.23 | 0.021 | 0.5 | 8.612 | 0.060 |
| 9 | 4 | 0 | 0 | 0 | 2 | 13.08 | 0.052 |
| 10 | 4 | 0 | 0 | 0.021 | 0.5 | 17.14 | 0.120 |
| 11 | 4 | 0 | 0.23 | 0 | 2 | 11.60 | 0.087 |
| 12 | 4 | 0 | 0.23 | 0.021 | 0.5 | 8.135 | 0.049 |
| 13 | 4 | 17.6 | 0 | 0 | 0.5 | 0.831 | 0.007 |
| 14 | 4 | 17.6 | 0 | 0.021 | 2 | 10.75 | 0.134 |
| 15 | 4 | 17.6 | 0.23 | 0 | 0.5 | 2.267 | 0.014 |
| 16 | 4 | 17.6 | 0.23 | 0.021 | 2 | 2.959 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, G.; Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Iqbal, H.; Parra-Saldívar, R. Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions. Polymers 2021, 13, 131. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010131
García G, Sosa-Hernández JE, Rodas-Zuluaga LI, Castillo-Zacarías C, Iqbal H, Parra-Saldívar R. Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions. Polymers. 2021; 13(1):131. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010131
Chicago/Turabian StyleGarcía, Gabriela, Juan Eduardo Sosa-Hernández, Laura Isabel Rodas-Zuluaga, Carlos Castillo-Zacarías, Hafiz Iqbal, and Roberto Parra-Saldívar. 2021. "Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions" Polymers 13, no. 1: 131. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010131