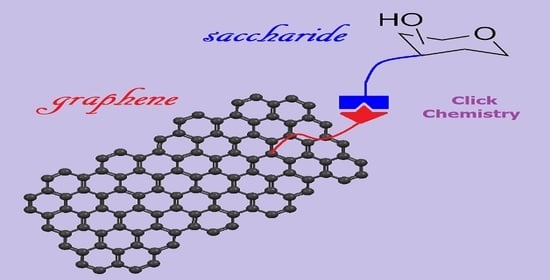
Click Chemistry Enabling Covalent and Non-Covalent Modifications of Graphene with (Poly)saccharides
Abstract
:1. Introduction
2. Functionalization Methodologies Involving Click Chemistry
3. Covalent Conjugation of (Poly)saccharides to Graphene
3.1. Poly- and Oligo-Saccharides
3.2. Mono and Di-Saccharides
4. Non-Covalent Conjugation of (Poly)saccharides to Graphene
5. Covalent Click Chemistry Approaches Applied to Other CRMs
6. Future Perspectives
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| CD | cyclodextrin |
| CNTs | Carbon Nanotubes |
| CRMs | Carbon Rich Materials |
| GO | Graphene Oxide |
| rGO | reduced Graphene Oxide |
| GBM | Graphene-Based Material |
| MWCNTs | Multi Walled Carbon Nanotubes |
| PSs | Polysaccharides |
References
- Slaven, G.; Hubbard, W.J.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J.A. Graphene as a subnanometre trans-electrode membrane. Nat. Cell Biol. 2010, 467, 190–193. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Georgakilas, V. (Ed.) Functionalization of Graphene; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; ISBN 9783527672790. [Google Scholar]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Khan, A.; Jawaid, M.; Neppolian, B.; Asiri, A.M. (Eds.) Graphene Functionalization Strategies; Carbon Nanostructures; Springer: Singapore, 2019; ISBN 978-981-32-9056-3. [Google Scholar]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, R.; Li, H.; Bergman, J.; Lundstedt, A.; Jorner, K.; Ayub, R.; Haldar, S.; Jahn, B.O.; Denisova, A.; Zietz, B.; et al. Metal-free photochemical silylations and transfer hydrogenations of benzenoid hydrocarbons and graphene. Nat. Commun. 2016, 7, 12962. [Google Scholar] [CrossRef]
- Lundstedt, A.; Papadakis, R.; Li, H.; Han, Y.; Jorner, K.; Bergman, J.; Leifer, K.; Grennberg, H.; Ottosson, H. White-Light Photoassisted Covalent Functionalization of Graphene Using 2-Propanol. Small Methods 2017, 1, 1700214. [Google Scholar] [CrossRef]
- Kansara, V.; Patil, R.; Tripathi, R.; Jha, P.K.; Bahadur, P.; Tiwari, S. Functionalized graphene nanosheets with improved dispersion stability and superior paclitaxel loading capacity. Colloids Surf. B 2019, 173, 421–428. [Google Scholar] [CrossRef]
- Li, H.; Papadakis, R.; Jafri, S.; Thersleff, T.; Michler, J.; Ottosson, H.; Leifer, K. Superior adhesion of graphene nanoscrolls. Commun. Phys. 2018, 1, 44. [Google Scholar] [CrossRef]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical Functionalization of Polysaccharides-Towards Biocompatible Hydrogels for Biomedical Applications. Chem.-A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I. Biocompatible injectable polysaccharide materials for drug delivery. In Polysaccharide Carriers for Drug Delivery; Elsevier BV: Geneva, Switzerland, 2019; pp. 127–154. [Google Scholar]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-Based Sensors for Human Health Monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Liu, H.; Li, T.; Liu, Y.; Qin, G.; Wang, X.; Chen, T. Glucose-Reduced Graphene Oxide with Excellent Biocompatibility and Photothermal Efficiency as well as Drug Loading. Nanoscale Res. Lett. 2016, 11, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Li, H.; Jafri, S.H.M.; Ossipov, D.; Hilborn, J.; Leifer, K. Optimization and analysis of pyrene-maltose functionalized graphene surfaces for Con A detection. Appl. Surf. Sci. 2020, 510, 145409. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Clarke, K.; Li, K. Covalent Functionalization of Graphene with Polysaccharides. Ind. Eng. Chem. Res. 2011, 51, 310–317. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymers 2013, 54, 5087–5103. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Cervantes, E.; López-Barroso, J.; Martínez-Hernández, A.L.; Velasco-Santos, C. Graphene-Based Materials Functionaliza-tion with Natural Polymeric Biomolecules. In Recent Advances in Graphene Research; IntechOpen: London, UK, 2016. [Google Scholar]
- Makvandi, P.; Ghomi, M.; Ashrafizadeh, M.; Tafazoli, A.; Agarwal, T.; Delfi, M.; Akhtari, J.; Zare, E.N.; Padil, V.V.; Zarrabi, A.; et al. A review on advances in graphene-derivative/polysaccharide bionanocomposites: Therapeutics, pharmacogenomics and toxicity. Carbohydr. Polym. 2020, 250, 116952. [Google Scholar] [CrossRef]
- Castelaín, M.; Martínez, G.; Marco, C.; Ellis, G.J.; Salavagione, H.J. Effect of Click-Chemistry Approaches for Graphene Modification on the Electrical, Thermal, and Mechanical Properties of Polyethylene/Graphene Nanocomposites. Macromolecules 2013, 46, 8980–8987. [Google Scholar] [CrossRef]
- Lai, C.; Sun, Y.; Yang, H.; Zhang, X.; Lin, B. The Functionalization of Graphene and Graphene Oxide via Click Chemistry. Acta Chim. Sin. 2013, 71, 901–1224. [Google Scholar] [CrossRef] [Green Version]
- Luong, N.D.; Sinh, L.H.; Johansson, L.-S.; Campell, J.; Seppälä, J. Functional Graphene by Thiol-ene Click Chemistry. Chem. -A Eur. J. 2015, 21, 3183–3186. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; González-Cortés, A.; Campuzano, S.; Pingarrón, J.M. Copper(I)-Catalyzed Click Chemistry as a Tool for the Functionalization of Nanomaterials and the Preparation of Electrochemical (Bio)Sensors. Sensors 2019, 19, 2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haley, M.M.; Tykwinski, R.R. (Eds.) Carbon-Rich Compounds; Wiley: Hoboken, NJ, USA, 2006; ISBN 9783527312245. [Google Scholar]
- Click Chemistry for Biotechnology and Materials Science. In Click Chemistry for Biotechnology and Materials Science; Wiley: Hoboken, NJ, USA, 2009.
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- De Alvarenga, E.S. Characterization and Properties of Chitosan, Biotechnology of Biopolymers; Elnashar, M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Lalov, I. Treatment of waste water from distilleries with chitosan. Water Res. 2000, 34, 1503–1506. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan grafted graphene oxide aerogel: Synthesis, characterization and carbon dioxide capture study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef]
- Ryu, H.J.; Mahapatra, S.S.; Yadav, S.K.; Cho, J.W. Synthesis of click-coupled graphene sheet with chitosan: Effective exfoliation and enhanced properties of their nanocomposites. Eur. Polym. J. 2013, 49, 2627–2634. [Google Scholar] [CrossRef]
- Kabiri, R.; Namazi, H. Surface grafting of reduced graphene oxide using nanocrystalline cellulose via click reaction. J. Nanopart. Res. 2014, 16, 2474. [Google Scholar] [CrossRef]
- Wong, C.-H.; Zimmerman, S.C. Orthogonality in organic, polymer, and supramolecular chemistry: From Merrifield to click chemistry. Chem. Commun. 2013, 49, 1679–1695. [Google Scholar] [CrossRef]
- Ye, Y.; Mao, X.; Xu, J.; Kong, J.; Hu, X. Functional Graphene Oxide Nanocarriers for Drug Delivery. Int. J. Polym. Sci. 2019, 2019, 8453493. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Lowry, M.; Fletcher, K.A.; Huang, X.; Powe, A.M.; Warner, I.M. Cyclodextrins Host- Guest Chemistry in Analytical and Environmental Chemistry. Curr. Anal. Chem. 2007, 3, 171–181. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Otero-Espinar, F.; Torres-Labandeira, J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Park, K.D.; Lee, Y.; Ryu, S.B.; Sung, H.-J.; Park, K.D. Oxidized cyclodextrin-functionalized injectable gelatin hydrogels as a new platform for tissue-adhesive hydrophobic drug delivery. RSC Adv. 2017, 7, 34053–34062. [Google Scholar] [CrossRef] [Green Version]
- Tasdelen, M.A. Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Gacal, B.; Durmaz, H.; Tasdelen, M.A.; Hizal, G.; Tunca, U.; Yagci, Y.; Demirel, A.L. Anthracene−Maleimide-Based Diels−Alder “Click Chemistry” as a Novel Route to Graft Copolymers. Macromolecules 2006, 39, 5330–5336. [Google Scholar] [CrossRef]
- Zhang, X.; Cong, Y.; Zhang, B. Reduced graphene oxide/liquid crystalline oligomer composites based on reversible covalent chemistry. Phys. Chem. Chem. Phys. 2017, 19, 6082–6089. [Google Scholar] [CrossRef]
- Munirasu, S.; Albuerne, J.; Boschetti-De-Fierro, A.; Abetz, V. Functionalization of Carbon Materials using the Diels-Alder Reaction. Macromol. Rapid Commun. 2010, 31, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yin, Z.; Wu, J. Covalent attachment of chitosan to graphene via click chemistry for superior antibacterial activity. Mater. Adv. 2020, 1, 579–583. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Cui, L.; Tang, J.; Liu, Z.; Kong, Q.; Yang, W.; Gooding, J.; Gooding, J.J. Using Molecular Level Modification to Tune the Conductivity of Graphene Papers. J. Phys. Chem. C 2012, 116, 17939–17946. [Google Scholar] [CrossRef]
- Zhu, Y.; James, D.K.; Tour, J.M. New Routes to Graphene, Graphene Oxide and Their Related Applications. Adv. Mater. 2012, 24, 4924–4955. [Google Scholar] [CrossRef]
- Golosova, A.A.; Papadakis, C.M.; Jordan, R. Chemical functionalization of carbon nanotubes with aryl diazonium salts. MRS Proc. 2011, 1362, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Namvari, M.; Namazi, H. Sweet graphene I: Toward hydrophilic graphene nanosheets via click grafting alkyne-saccharides onto azide-functionalized graphene oxide. Carbohydr. Res. 2014, 396, 1–8. [Google Scholar] [CrossRef]
- Tornoe, C.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Namazi, H.; Namazi, H. Preparation of efficient magnetic biosorbents by clicking carbohydrates onto graphene oxide. J. Mater. Sci. 2015, 50, 5348–5361. [Google Scholar] [CrossRef]
- Speranza, M. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C—J. Carbon Res. 2019, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, I.; Barras, A.; Coffinier, Y.; Lisowski, W.; Roy, S.; Niedziolka-Jonsson, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Siriwardena, A.; et al. Preparation of a Responsive Carbohydrate-Coated Biointerface Based on Graphene/Azido-Terminated Tetrathiafulvalene Nanohybrid Material. ACS Appl. Mater. Interfaces 2012, 4, 5386–5393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Wei, X.-L.; Zang, Y.; Cao, J.-Y.; Liu, S.; He, X.-P.; Chen, Q.; Long, Y.-T.; Li, J.; Chen, G.-R.; et al. Fluorogenic Probing of Specific Recognitions between Sugar Ligands and Glycoprotein Receptors on Cancer Cells by an Economic Graphene Nanocomposite. Adv. Mater. 2013, 25, 4097–4101. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Leal, M.P.; Fernández, I.; Romero-Gomez, P.; Baati, R.; Khiar, N. Improved non-covalent biofunctionalization of multi-walled carbon nanotubes using carbohydrate amphiphiles with a butterfly-like polyaromatic tail. Nano Res. 2010, 3, 764–778. [Google Scholar] [CrossRef] [Green Version]
- Gingras, M.; Chabre, Y.M.; Roy, M.; Roy, R. How do multivalent glycodendrimers benefit from sulfur chemistry? Chem. Soc. Rev. 2013, 42, 4823–4841. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.; Hu, N.; Tam, U.C.; Blixt, O.; Zettl, A.; Bertozzi, C.R. Biocompatible Carbon Nanotubes Generated by Functionalization with Glycodendrimers. Angew. Chem. Int. Ed. 2008, 47, 5022–5025. [Google Scholar] [CrossRef]
- Ji, X.; Cuib, L.; Xu, Y.; Liu, J. Non-covalent interactions for synthesis of new graphene based composites. Compos. Sci. Technol. 2015, 106, 25–31. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Pykal, M.; Jurečka, P.; Karlický, F.; Otyepka, M. Modelling of graphene functionalization. Phys. Chem. Chem. Phys. 2016, 18, 6351–6372. [Google Scholar] [CrossRef] [Green Version]
- Lundstedt, A. Development of Mild Methods for Selective Covalent Functionalization of Graphene. Ph.D. Thesis, Uppsala University, Stockholm, Sweden, 2017. [Google Scholar]
- He, X.-P.; Zang, Y.; James, T.D.; Li, J.; Chen, D.; Xie, J. Fluorescent glycoprobes: A sweet addition for improved sensing. Chem. Commun. 2017, 53, 82–90. [Google Scholar] [CrossRef] [Green Version]
- He, X.-P.; Li, R.-H.; Maisonneuve, S.; Ruan, Y.; Chen, G.-R.; Xie, J. Fluorogenic supramolecular complexes formed between pyrenyl-β-cyclodextrin and glyco-rhodamine for the selective detection of lectins. Chem. Commun. 2014, 50, 14141–14144. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mandal, D.; Deb, P.; Mondal, B.; Ghosh, S. Synthesis of triazole linked fluorescent amino acid and carbohydrate bio-conjugates: A highly sensitive and skeleton selective multi-responsive chemosensor for Cu(ii) and Pb(ii)/Hg(ii) ions. RSC Adv. 2014, 4, 1918–1928. [Google Scholar] [CrossRef]
- He, X.; Xie, J.; Chen, G.-R.; Chen, K. Pyrene Excimer-based Bis-triazolyl Pyranoglycoligands as Specific Mercury Sensors. Chin. J. Chem. 2012, 30, 2874–2878. [Google Scholar] [CrossRef]
- Guldi, D.M.; Martín, N. (Eds.) Carbon Nanotubes and Related Structures. Synthesis, Characterization, Functionalization, and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cheng, F.; Duft, A.M.; Adronov, A. Functionalization of Single-Walled Carbon Nanotubes with Well-Defined Polystyrene by “Click” Coupling. J. Am. Chem. Soc. 2005, 127, 14518–14524. [Google Scholar] [CrossRef]
- Li, H.; Adronov, A. Water-soluble SWCNTs from sulfonation of nanotube-bound polystyrene. Carbon 2007, 45, 984–990. [Google Scholar] [CrossRef]
- Guo, Z.; Liang, L.; Liang, J.-J.; Ma, Y.-F.; Yang, X.-Y.; Ren, D.-M.; Chen, Y.-S.; Zheng, J.-Y. Covalently β-cyclodextrin modified single-walled carbon nanotubes: A novel artificial receptor synthesized by ‘click’ chemistry. J. Nanopart. Res. 2008, 10, 1077–1083. [Google Scholar] [CrossRef]
- Fan, Z.; Diao, C.-H.; Song, H.-B.; Jing, Z.-L.; Yu, M.; Chen, X.; Guo, M.-J. Encapsulation of Quinine by β-Cyclodextrin: Excellent Model for Mimicking Enzyme−Substrate Interactions. J. Org. Chem. 2006, 71, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Mahapatra, S.S.; Yadav, M.K.; Dutta, P.K. Mechanically robust biocomposite films of chitosan grafted carbon nanotubes via the [2 + 1] cycloaddition of nitrenes. RSC Adv. 2013, 3, 23631. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; He, H.; Zhou, L.; Zheng, X.; Zhang, Y. Scalable Functional Group Engineering of Carbon Nanotubes by Improved One-Step Nitrene Chemistry. Chem. Mater. 2009, 21, 360–370. [Google Scholar] [CrossRef]
- Setaro, A.; Adeli, M.; Glaeske, M.; Przyrembel, D.; Bisswanger, T.; Gordeev, G.; Maschietto, F.; Faghani, A.; Paulus, B.; Weinelt, M.; et al. Preserving π-conjugation in covalently functionalized carbon nanotubes for optoelectronic applications. Nat. Commun. 2017, 8, 14281. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Papadakis, R. Click Chemistry Enabling Covalent and Non-Covalent Modifications of Graphene with (Poly)saccharides. Polymers 2021, 13, 142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010142
Li H, Papadakis R. Click Chemistry Enabling Covalent and Non-Covalent Modifications of Graphene with (Poly)saccharides. Polymers. 2021; 13(1):142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010142
Chicago/Turabian StyleLi, Hu, and Raffaello Papadakis. 2021. "Click Chemistry Enabling Covalent and Non-Covalent Modifications of Graphene with (Poly)saccharides" Polymers 13, no. 1: 142. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010142