Robust Effects of Graphene Oxide on Polyurethane/Tourmaline Nanocomposite Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PU/TN and PU/TN/GO
2.3. Preparation of Wet Spinning Fibers
2.4. Characterization of Wet Spinning Fibers
3. Results and Discussion
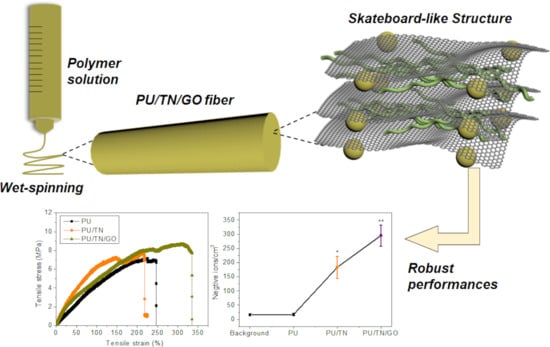
3.1. Structures and Morphologies of PU and Its Nanocomposites Fibers
3.2. Thermal and Mechanical Properties of PU and Its Nanocomposites Fibers
3.3. Negative Ions Releasing Performances
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Li, Y. The Function and Health Care of Negative Ion Fibers and Textiles. In Proceedings of the 2010 International Conference on Information Technology and Scientific Management, Tianjin, China, 20–21 December 2010. [Google Scholar]
- Kim, S.H.; Hwang, S.H.; Hong, S.K.; Seo, J.K.; Sung, H.S.; Park, S.W.; Shin, J.H. The clinical efficacy, safety and functionality of anion textile in the treatment of atopic dermatitis. Ann. Dermatol. 2012, 24, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirillova, A.; Kelly, C.; von Windheim, N.; Gall, K. Biomaterials: Bioinspired Mineral–Organic Bioresorbable Bone Adhesive (Adv. Healthcare Mater. 17/2018). Adv. Healthc. Mater. 2018, 7, 1870070. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Biedrzycki, A.H.; Khalil, A.S.; Hess, D.; Umhoefer, J.M.; Markel, M.D.; Murphy, W.L. Nanostructured mineral coatings stabilize proteins for therapeutic delivery. Adv. Mater. 2017, 29, 1701255. [Google Scholar] [CrossRef] [PubMed]
- Ryushi, T.; Kita, I.; Sakurai, T.; Yasumatsu, M.; Isokawa, M.; Aihara, Y.; Hama, K. The effect of exposure to negative air ions on the recovery of physiological responses after moderate endurance exercise. Int. J. Biometeorol. 1998, 41, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Nakane, H.; Asami, O.; Yamada, Y.; Ohira, H. Effect of negative air ions on computer operation, anxiety and salivary chromogranin A-like immunoreactivity. Int. J. Psychophysiol. 2002, 46, 85–89. [Google Scholar] [CrossRef]
- Bajirova, M. Miraculous effects of Negative Ions on Urogenital Infections. Obstet. Gynecol. Int. J. 2018, 9, 00297. [Google Scholar] [CrossRef] [Green Version]
- Liko, I.; Hopper, J.T.; Allison, T.M.; Benesch, J.L.; Robinson, C.V. Negative ions enhance survival of membrane protein complexes. J. Am. Soc. Mass Spectrom. 2016, 27, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safak, S.; Karaca, E. Production and characterization of poly (ethylene terephthalate) nanofibrous mat including tourmaline additive. Text. Res. 2016, 86, 1651–1658. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Li, J.; Zhu, Y.; Ge, M. Negative air ion release and far infrared emission properties of polyethylene terephthalate/germanium composite fiber. J. Eng. Fibers Fabr. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Hui, N.; Zhao, T.; Zhang, H. The mineralization of polymer electrospun fibrous membranes modified with tourmaline nanoparticles. J. Mater. Res. 2019, 34, 1900–1910. [Google Scholar] [CrossRef]
- Skogby, H.; Bosi, F.; Lazor, P. Short-range order in tourmaline: A vibrational spectroscopic approach to elbaite. Phys. Chem. Miner. 2012, 39, 811–816. [Google Scholar] [CrossRef]
- Tijing, L.D.; Ruelo, M.T.G.; Amarjargal, A.; Pant, H.R.; Park, C.-H.; Kim, D.W.; Kim, C.S. Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem. Eng. J. 2012, 197, 41–48. [Google Scholar] [CrossRef]
- Tijing, L.D.; Amarjargal, A.; Jiang, Z.; Ruelo, M.T.G.; Park, C.-H.; Pant, H.R.; Kim, D.-W.; Lee, D.H.; Kim, C.S. Antibacterial tourmaline nanoparticles/polyurethane hybrid mat decorated with silver nanoparticles prepared by electrospinning and UV photoreduction. Curr. Appl. Phys. 2013, 13, 205–210. [Google Scholar] [CrossRef]
- Pengyu, B. A study of textiles of negative ions and their application. Indian J. Text. Res. 2003, 24, 99–101. [Google Scholar]
- Wang, C.; Liu, J.; Zhang, Z.; Wang, B.; Sun, H. Adsorption of Cd(II), Ni(II), and Zn(II) by tourmaline at acidic conditions: Kinetics, thermodynamics, and mechanisms. Ind. Eng. Chem. Res. 2012, 51, 4397–4406. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X. The surface organic modification of tourmaline powder by span-60 and its composite. Appl. Surf. Sci. 2012, 258, 7540–7545. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J.; Meng, Q. Nonwoven supported temperature-sensitive poly (N-isopropylacrylamide)/polyurethane copolymer hydrogel with antibacterial activity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.; Chen, S.; Ji, F. Theoretical study of hydrogen bonding interactions on MDI-based polyurethane. J. Mol. Model. 2010, 16, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Lin, T. Wound dressing. Mater. Lett. 2015, 156, 46–49. [Google Scholar]
- Zhang, Y.; Hu, J.; Zhao, X.; Xie, R.; Qin, T.; Ji, F. Mechanically Robust Shape Memory Polyurethane Nanocomposites for Minimally Invasive Bone Repair. ACS Appl. Bio Mater. 2019, 2, 1056–1065. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Barakat, N.A.; Kanjwal, M.A.; Chaudhari, A.A.; Jung, I.-H.; Lee, J.H.; Kim, H.Y. Electrospun antimicrobial polyurethane nanofibers containing silver nanoparticles for biotechnological applications. Macromol. Res. 2009, 17, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Si, S.; Chen, Y.; Yuan, T.; Fan, H.; Yao, Y.; Zhang, Q. Electrospun in-situ hybrid polyurethane/nano-TiO 2 as wound dressings. Fibers Polym. 2011, 12, 207–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhu, S.; Qin, T.; Ji, F. A “trampoline” nanocomposite: Uning the interlayer spacing in graphene oxide/polyurethane to achieve coalesced mechanical and memory. Compos. Sci. Technol. 2019, 180, 14–22. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Zhang, S.Y.; Li, Y.J.; Sun, J.; Qin, C.X.; Wang, J.J.; Dai, L.X. Modification of GO based on click reaction and its composite fibers with poly(vinyl alcohol). Compos. Part A Appl. Sci. 2017, 101, 115–122. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Meng, X.Y.; Han, Y.S.; Ye, H.M.; Cui, L.S.; Zhou, Q. Reinforcing polyamide 1212 with graphene oxide via a two-step melt compounding process. Compos. Part A Appl. Sci. 2015, 69, 115–123. [Google Scholar] [CrossRef]
- Guan, L.-Z.; Zhao, L.; Wan, Y.-J.; Tang, L.-C. Three-dimensional graphene-based polymer nanocomposites: Preparation, properties and applications. Nanoscale 2018, 10, 14788–14811. [Google Scholar] [CrossRef]
- Pokharel, P. High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol. Chem. Eng. J. 2014, 253, 356–365. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Hu, D.; Xiao, C.; Zhuo, Q.; Wang, J.; Qin, C.; Dai, L. Large-sized graphene oxide/modified tourmaline nanoparticle aerogel with stable honeycomb-like structure for high-efficiency PM 2.5 capture. J. Mater. Chem. A 2018, 6, 16139–16148. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Guo, T.; Li, Q. Environmental effects and enhancement mechanism of graphene/tourmaline composites. J. Clean. Prod. 2020, 262, 121313. [Google Scholar] [CrossRef]
- Xue, G.; Luo, X.; Srinivasakannan, C.; Zheng, L.; Miao, Y.; Xinhui, D. Effective removal of organic dye and heavy metal from wastewater by tourmaline/graphene oxide composite nano material. Mater. Res. Express 2019, 6, 115618. [Google Scholar] [CrossRef]
- Shi, S.; Han, Y.; Hu, J. Robust waterproof and self-adaptive breathable membrane with heat retention property for intelligent protective cloth. Prog. Org. Coat. 2019, 137, 105303. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Jin, W.; Liang, J.; Ding, Y.; Gan, K.; Yuan, Y. Effects of particle size on far infrared emission properties of tourmaline superfine powders. J. Nanosci. Nanotechnol. 2010, 10, 2083–2087. [Google Scholar] [CrossRef]
- Wang, Y.; Yeh, J.T.; Yue, T.; Chiu, Y.H.; Shen, X. Influence of tourmaline on negative air ion emitting property of poly (ethylene terephthalate). J. Macromol. Sci. Part A Pure Appl. Chem. 2006, 43, 1749–1756. [Google Scholar] [CrossRef]
- Mazzoli, A.; Corinaldesi, V.; Donnini, J.; Di Perna, C.; Micheli, D.; Vricella, A.; Pastore, R.; Bastianelli, L.; Moglie, F.; Primiani, V.M. Effect of graphene oxide and metallic fibers on the electromagnetic shielding effect of engineered cementitious composites. JOBE 2018, 18, 33–39. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, K.H.; Yadav, S.K.; Cho, J.W. Effects of carbon nanotube functionalization and annealing on crystallization and mechanical properties of melt-spun carbon nanotubes/poly(ethylene terephthalate) fibers. Compos. Sci. Technol. 2012, 72, 1834–1840. [Google Scholar] [CrossRef]
- Yu, J.; Xia, H.; Teramoto, A.; Ni, Q.Q. The effect of hydroxyapatite nanoparticles on mechanical behavior and biological performance of porous shape memory polyurethane scaffolds. J. Biomed. Mater. Res. Part A 2018, 106, 244–254. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Wang, C.; Guo, T. Preparation and improved negative ion release of graphene/tourmaline composite. Mater. Res. Express 2019, 6, 055507. [Google Scholar] [CrossRef]






| Items | PU | PU/TN | PU/TN/GO |
|---|---|---|---|
| Tensile strength (MPa) | 7.0 | 7.7 | 8.7 |
| Tensile strain at break (%) | 247 | 215 | 335 |
| Young’s modulus (MPa) | 4.3 | 8.3 | 8.4 |
| Maximum Load at break (N) | 0.08 | 0.05 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, J. Robust Effects of Graphene Oxide on Polyurethane/Tourmaline Nanocomposite Fiber. Polymers 2021, 13, 16. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010016
Zhang Y, Hu J. Robust Effects of Graphene Oxide on Polyurethane/Tourmaline Nanocomposite Fiber. Polymers. 2021; 13(1):16. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010016
Chicago/Turabian StyleZhang, Yuanchi, and Jinlian Hu. 2021. "Robust Effects of Graphene Oxide on Polyurethane/Tourmaline Nanocomposite Fiber" Polymers 13, no. 1: 16. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010016