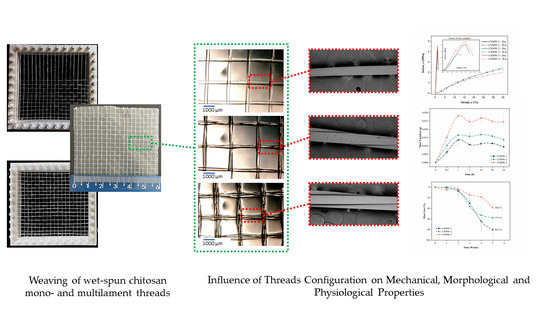
Chitosan Woven Meshes: Influence of Threads Configuration on Mechanical, Morphological, and Physiological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan Threads Preparation
2.3. Chitosan Woven Meshes Preparation
2.4. Optical Microscopy (OM)
2.5. Scanning Electron Microscopy (SEM)
2.6. Mechanical Properties
2.7. Swelling Test
2.8. In Vitro Enzymatic Degradation
2.9. Statistical Evaluation
3. Results and Discussion
3.1. Threads Characterization
3.1.1. Morphological Analysis
3.1.2. Mechanical Properties
3.2. Woven Meshes Characterization
3.2.1. Morphological Analysis
3.2.2. Mechanical Properties
3.2.3. Swelling Tests
3.2.4. In Vitro Enzymatic Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of chitosan for biomedical applications. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–30. [Google Scholar]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Agboh, O.C.; Qin, Y. Chitin and Chitosan Fibers. Polym. Adv. Technol. 1997, 8, 355–365. [Google Scholar] [CrossRef]
- East, G.C.; Qin, Y. Wet spinning of chitosan and the acetylation of chitosan fibers. J. Appl. Polym. Sci. 1993, 50, 1773–1779. [Google Scholar] [CrossRef]
- Felinto, M.C.F.C.; Parra, D.F.; Da Silva, C.C.; Angerami, J.; Oliveira, M.J.A.; Lugão, A.B. The swelling behavior of chitosan hydrogels membranes obtained by UV- and γ-radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 418–424. [Google Scholar] [CrossRef]
- Ozipek, B.; Karakas, H. Wet spinning of synthetic polymer fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 174–186. [Google Scholar]
- Ravi Kumar, M.N.V. Chitin and chitosan fibres: A review. Bull. Mater. Sci. 1999, 22, 905. [Google Scholar] [CrossRef]
- King, M.W.; Chung, S. Medical Fibers and Biotextiles. In Biomaterials Science, 3rd ed.; Ratner, B., Hoffman, A., Schoen, F., Lemons, J., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 301–320. [Google Scholar]
- Yu, S.; Ma, P.; Cong, H.; Jiang, G. Preparation and Performances of Warp-Knitted Hernia Repair Mesh Fabricated with Chitosan Fiber. Polymers 2019, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Sumanasingh, R.; King, M.W. The Applications of Biotextiles in Tissue Engineering. Res. J. Text. Appar. 2005, 9, 80–90. [Google Scholar] [CrossRef]
- Sumanasinghe, R.D.; King, M.W. New trends in biotextiles—the challenge of tissue engineering. J. Text. Appar. Technol. Manag. 2003, 3, 1–13. [Google Scholar]
- Aghaei-Ghareh-Bolagh, B.; Mithieux, S.M.; Hiob, M.A.; Wang, Y.; Chong, A.; Weiss, A.S. Fabricated tropoelastin-silk yarns and woven textiles for diverse tissue engineering applications. Acta. Biomater. 2019, 91, 112–122. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Wang, X.; Zheng, Y.; Han, C. Three types of dermal grafts in rats: The importance of mechanical property and structural design. Biomed. Eng. OnLine 2013, 12, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, N.; Kasahara, Y.; Yamane, S.; Igarashi, T.; Minami, A.; Nisimura, S.I. Chitosan-Based Hyaluronic Acid Hybrid Polymer Fibers as a Scaffold Biomaterial for Cartilage Tissue Engineering. Polymers 2011, 3, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; You, C.; Hu, X.; Zheng, Y.; Li, Q.; Feng, Z.; Sun, H.; Gao, C.; Han, C. The roles of knitted mesh-reinforced collagen-chitosan hybrid scaffold in the one-step repair of full-thickness skin defects in rats. Acta Biomater. 2013, 9, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.R.; Martins, A.R.; Fernandes, E.M.; Oliveira, M.B.; Correlo, V.M.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Durães, N.F.; Silva, C.J.; et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater. 2013, 9, 8167–8181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P.; Hu, X.; You, C.; Guo, R.; Shi, H.; Guo, S.; Zhou, H.; Chaoheng, Y.; Zhang, Y.; et al. Polyurethane membrane/knitted mesh-reinforced collagen–chitosan bilayer dermal substitute for the repair of full-thickness skin defects via a two-step procedure. J. Mech. Behav. Biomed. Mater. 2016, 56, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Wawro, D.; Skrzetuska, E.; Włodarczyk, B.; Kowalski, K.; Krucińska, I. Processing of Chitosan Yarn into Knitted Fabrics. Fibres Text. East. Eur. 2016, 6, 52–57. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Leal, R.D.C.A.; Da Silva, H.N.; Fook, M.V.L. Biodegradable suture threads as controlled drug delivery systems. Mater. Res. Innov. 2019, 24, 1–5. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Da Silva, H.N.; Holanda, S.A.; Silva, A.R.O.; Fook, M.V.L. Biodegradable polymeric wires: Monofilament and multifilament. Mater. Res. Innov. 2019, 24, 161–165. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Da Silva, H.N.; Alves Leal Cruz, R.d.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials 2019, 12, 1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panar, M.; Avakian, P.; Blume, R.C.; Gardner, K.H.; Gierke, T.D.; Yang, H.H. Morphology of poly(p-phenylene terephthalamide) fibers. J. Polym. Sci. Polym. Physics Ed. 1983, 21, 1955–1969. [Google Scholar] [CrossRef]
- Dawelbeit, A.; Hongpeng, Z.; Haijuan, K.; Jing, L.; Yu, M.; Muhuo, Y. Microstructural Developments of Poly(p-phenylene terephthalamide) Fibers During Heat Treatment Process: A Review. Mater. Res. 2014, 17, 1180–1200. [Google Scholar]
- Yang, H.M. Aramid Fibers. In Comprehensive Composite Materials; Kelly, A., Zweben, C., Eds.; Pergamon: Oxford, UK, 2000; pp. 199–229. [Google Scholar]
- Chawla, K.K. Fibrous Materials; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Gafurov, J.K.; Mardonov, B.; Gofurov, K.; Dushamov, O.S.; Ergashev, O.O.; Bobajonov, H.T. Yarn Deformation with view of its structural structure. MS&E 2018, 459, 012042. [Google Scholar]
- Wu, T.; Farnood, R.; O’Kelly, K.; Chen, B. Mechanical behavior of transparent nanofibrillar cellulose–chitosan nanocomposite films in dry and wet conditions. J. Mech. Behav. Biomed. Mater. 2014, 32, 279–286. [Google Scholar] [CrossRef]
- Blasi, P.; D’Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly(lactide-co-glycolide). J. Control. Release 2005, 108, 1–9. [Google Scholar] [CrossRef]
- Alamri, H.; Low, I.M. Effect of water absorption on the mechanical properties of nano-filler reinforced epoxy nanocomposites. Mater. Des. 2012, 42, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Remuñán-López, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Yin, Y.; Yao, F.; Yao, K. Modulation of nano-hydroxyapatite size via formation on chitosan–gelatin network film in situ. Biomater. 2007, 28, 781–790. [Google Scholar] [CrossRef]
- Oh, D.X.; Hwang, D.S. A biomimetic chitosan composite with improved mechanical properties in wet conditions. Biotechnol. Prog. 2013, 29, 505–512. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Hu, X.; Ma, L.; You, C.; Zheng, Y.; Sun, H.; Han, C.; Gao, C. Fabrication and characterization of poly(L-lactide-co-glycolide) knitted mesh-reinforced collagen–chitosan hybrid scaffolds for dermal tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 8, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.; Baldeweck, T.; Tanter, M.; Catheline, S.; Fink, M.; Sandrin, L.; Cornillon, C.; Querleux, B. Assessment of elastic parameters of human skin using dynamic elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.-D.; Liu, J.; Cheng, G.-X.; Zhao, R.-Z.; Wang, W.H.; Wei, L. The dynamic swelling behaviour of chitosan-based hydrogels. Polym. Int. 1998, 45, 191–194. [Google Scholar] [CrossRef]
- Yodkhum, K.; Phaechamud, T. Hydrophobic chitosan sponges modified by aluminum monostearate and dehydrothermal treatment as sustained drug delivery system. Mater. Sci. Eng. C 2014, 42, 715–725. [Google Scholar] [CrossRef]
- Khalid, M.N.; Agnely, F.; Yagoubi, N.; Grossiord, J.L.; Couarraze, G. Water state characterization, swelling behavior, thermal and mechanical properties of chitosan based networks. Eur. J. Pharm. Sci. 2002, 15, 425–432. [Google Scholar] [CrossRef]
- Etienne, O.; Schneider, A.; Taddei, C.; Richert, L.; Schaaf, P.; Voegel, J.-C.; Egles, C.; Picart, C. Degradability of Polysaccharides Multilayer Films in the Oral Environment: an in Vitro and in Vivo Study. Biomacromolecules 2005, 6, 726–733. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Neamnark, A.; Sanchavanakit, N.; Pavasant, P.; Bunaprasert, T.; Supaphol, P.; Rujiravanit, R. In vitro biocompatibility evaluations of hexanoyl chitosan film. Carbohydr. Polym. 2007, 68, 166–172. [Google Scholar] [CrossRef]
- Tanuma, H.; Saito, T.; Nishikawa, K.; Dong, T.; Yazawa, K.; Inoue, Y. Preparation and characterization of PEG-cross-linked chitosan hydrogel films with controllable swelling and enzymatic degradation behavior. Carbohydr. Polym. 2010, 80, 260–265. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.K.; Wu, S.J.; Kim, J.M. Preparation of glucosamine by hydrolysis of chitosan with commercial α-amylase and glucoamylase. J. Zhejiang Univ. Sci. B 2011, 12, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Shin, H.-D.; Chen, R.; Li, J.; Du, G.; Chen, J. Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L. Glucosamine: An ingredient with skin and other benefits. J. Cosmet. Dermatol. 2006, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef] [PubMed]









| Thread Configuration | Diameter (µm) | Twist Angle (°) | Porosity (%) | Average Ore Size (µm) |
|---|---|---|---|---|
| Monofilament | 171 ± 2 (A) | – | 1.1 ± 0.89 (A) | 0.66 ± 0.257 (A) |
| Two-filaments | 260 ± 13 (B) | 5.3 ± 0.30 (A) | 2.7 ± 1.75 (B) | 0.82 ± 0.230 (A) |
| Three-filament | 312 ± 22 (C) | 3.7 ± 0.92 (B) | 1.8 ± 0.57 (A, B) | 0.72 ± 0.274 (A) |
| Thread Configuration | Tensile Strength (MPa) | Elastic Module (Gpa) | Strain (%) |
|---|---|---|---|
| Monofilament | 171 ± 22.26 (A) | 8 ± 0.93 (A) | 7 ± 2.23 (A) |
| Two-filament | 127 ± 7.82 (B) | 6 ± 0.26 (B) | 8 ± 2.29 (A) |
| Three-filament | 230 ± 24.38 (C) | 11 ± 0.72 (C) | 10 ± 2.3 (A) |
| Woven Meshes Code | Thread Configuration |
|---|---|
| CSWM-1 | Monofilament |
| CSWM-2 | Two-filament |
| CSWM-3 | Three-filament |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, H. N.; da Silva, M. C.; dos Santos, F. S.F.; da Silva Júnior, J. A.C.; Barbosa, R. C.; Fook, M. V.L. Chitosan Woven Meshes: Influence of Threads Configuration on Mechanical, Morphological, and Physiological Properties. Polymers 2021, 13, 47. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010047
da Silva HN, da Silva MC, dos Santos FSF, da Silva Júnior JAC, Barbosa RC, Fook MVL. Chitosan Woven Meshes: Influence of Threads Configuration on Mechanical, Morphological, and Physiological Properties. Polymers. 2021; 13(1):47. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010047
Chicago/Turabian Styleda Silva, Henrique Nunes, Milena Costa da Silva, Flavia Suzany Ferreira dos Santos, José Alberto Campos da Silva Júnior, Rossemberg Cardoso Barbosa, and Marcus Vinícius Lia Fook. 2021. "Chitosan Woven Meshes: Influence of Threads Configuration on Mechanical, Morphological, and Physiological Properties" Polymers 13, no. 1: 47. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010047