Color Fixation Strategies on Sustainable Poly-Butylene Succinate Using Biobased Itaconic Acid
Abstract
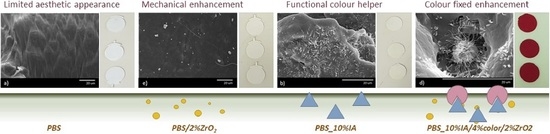
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nano-Bio-Composites Preparation
2.3. General Characterisation Methods
2.3.1. Two Types of Specimens Were Developed for Material Characterization
- Injected specimens for mechanical (tensile or dog-bone following ISO 178 standard) and hardness (parallelepiped specimens of 80 × 100 × 4 mm) testing were obtained by injection molding with a JSW 85 EL II electric injection machine.
- Circular specimens of 50 mm diameter and 2 mm thick from all the formulations were produced for measuring wettability and color change. The same processing parameters were considered. The materials were mixed for 120 s at 90 rpm in a co-rotating twin-screw extruder Microcompounder at 5 and 15 cc, DSM (Sittard, The Netherlands), using a temperature profile of 120–125–130 °C. Due to the low viscosity of the formulations containing IA, a pressure-time injection molding profile of 1.0–5; 1.1–15; 1.1–15 in bar-seconds was used. The mold and injection temperatures were set at 30 and 150 °C, respectively.
2.3.2. Measurements
3. Results and Discussion
3.1. Mechanical Tests
3.1.1. Comparative Results among Formulations at W0 (Comparisons among Pink Columns—Young′s Modulus; and among Green Columns—Elongation at Break)
3.1.2. Comparative Results of Same Formulations between W0 and W4 (Aging Effect)
3.2. Hardness
3.2.1. Hardness Results at W0
3.2.2. Hardness Results at W4
3.3. Structural Properties (SEM)
3.3.1. Structural Results at W0
3.3.2. Structural Results at W4
3.4. Hydrophobicity/Wettability
3.4.1. Structural Results at W0
3.4.2. Structural Results at W4
3.5. Color Properties
3.5.1. Color Results at W0
3.5.2. Color Results at W4
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resch-Fauster, K.; Klein, A.; Blees, E.; Feuchter, M. Mechanical recyclability of technical biopolymers: Potential and limits. Polym. Test. 2017, 64, 287–295. [Google Scholar] [CrossRef]
- Ma, P. Tailoring the Properties of Bio-Based and Biocompostable Polymer Blend. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2011. [Google Scholar]
- Totaro, G.; Sisti, L.; Celli, A.; Askanian, H.; Hennous, M.; Verney, V.; Leroux, F. Chain extender effect of 3-(4-hydroxyphenyl)propionic acid/layered double hydroxide in PBS bionanocomposites. Eur. Polym. J. 2017, 94, 20–32. [Google Scholar] [CrossRef]
- Rudnik, E. Properties and applications. Compost. Polym. Mater. 2019, 49–98. [Google Scholar] [CrossRef]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Joy, J.; Jose, C.; Varanasi, S.B.; Thomas, S.; Pilla, S. Preparation and Characterization of Poly(butylene succinate) Bionanocomposites Reinforced with Cellulose Nanofiber Extracted from Helicteres isora Plant. J. Renew. Mater. 2016, 4, 351–364. [Google Scholar] [CrossRef]
- Śmigiel-Gac, N.; Pamuła, E.; Krok-Borkowicz, M.; Smola-Dmochowska, A.; Dobrzyński, P. Synthesis and Properties of Bioresorbable Block Copolymers of l-Lactide, Glycolide, Butyl Succinate and Butyl Citrate. Polymers 2020, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A. The Effect of Dyes, Pigments and Ionic Liquids on the Properties of Elastomer Composites. Polymers. Ph.D. Thesis, Université Claude Bernard-Lyon I, Villeurbanne, France, Uniwersytet lódzki Lodz, Łódźc, Poland, 2014. [Google Scholar]
- Tolinski, M. Colorants. Addit. Polyolefins 2009, 137–156. [Google Scholar] [CrossRef]
- Püntener, A.; Page, C. European Ban on Certain Azo Dyes, Quality and Environment; TFL: Jalandhar, India, 2012. [Google Scholar]
- Lassen, P. Description of development of an analytical method for measurement of PAA in tattoo ink and PMU. In The Danish Environmental Protection Agency; Aarhus University: Aarhus, Denmark, 2017; ISBN 978-87-93614-03-1. [Google Scholar]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Heer, K.; Sharma, S. Microbial pigments as a natural color: A review. Int. J. Pharm. Sci. Res. 2017, 8, 1913–1922. [Google Scholar] [CrossRef]
- Fernández-López, J.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Das, D.; Datta, D.B.; Bhattacharya, P. Simultaneous Dyeing and Finishing of Silk Fabric with Natural Color and Itaconic Acid. Cloth. Text. Res. J. 2014, 32, 93–106. [Google Scholar] [CrossRef]
- Aromatic Azo- and Benzidine-Based Substances. Draft Technical Background Document. The Chemicals Management Plan Substance Groupings Initiative. Environment Canada. Health Canada, July 2012. Available online: https://www.canada.ca/en/health-canada/services/chemical-substances/substance-groupings-initiative/aromatic-azo-benzidine-based.html (accessed on 24 November 2020).
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef] [Green Version]
- Praveen Kumar, R.; Gnansounou, E.; Kenthorai Raman, J.; Baskar, G. Refining Biomass Residues for Sustainable Energy and Bioproducts. Technol. Adv. Life Cycle Assess. Econ. 2019. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Magalhaes, A.; Soccol, C. Biobased itaconic acid market and research trends - is it really a promising chemical? Chim. Oggi Chem. Today 2018, 36, 56. [Google Scholar]
- Teleky, B.-E.; Vodnar, D. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Peinado, V.; García, L.; Fernández, Á.; Castell, P. Novel lightweight foamed poly(lactic acid) reinforced with different loadings of functionalised Sepiolite. Compos. Sci. Technol. 2014, 101, 17–23. [Google Scholar] [CrossRef]
- García, L.; Fernández, Á; Castell, P.; García-Quiles, L. Sustainable Materials with Enhanced Mechanical Properties Based on Industrial Polyhydroxyalkanoates Reinforced with Organomodified Sepiolite and Montmorillonite. Polymers 2019, 11, 696. [Google Scholar] [CrossRef] [Green Version]
- Szeluga, U.; Kumanek, B.; Trzebicka, B. Synergy in hybrid polymer/nanocarbon composites. A review. Compos. Part A Appl. Sci. Manuf. 2015, 73, 204–231. [Google Scholar] [CrossRef]
- Mirica, I.C.; Furtos, G.; Bâldea, B.; Lucaciu, O.P.; Ilea, A.; Moldovan, M.; Campian, R.S. Influence of Filler Loading on the Mechanical Properties of Flowable Resin Composites. Materials 2020, 13, 1477. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef]
- Daou, E.E. The Zirconia Ceramic: Strengths and Weaknesses. Open Dent. J. 2014, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Özkurt-Kayahan, Z. Monolithic zirconia: A review of the literatura. Biomed. Res. 2016, 27, 4. [Google Scholar]
- Rahaman, M.N.; Li, Y.; Bal, B.S.; Huang, W. Functionally graded bioactive glass coating on magnesia partially stabilized zirconia (Mg-PSZ) for enhanced biocompatibility. J. Mater. Sci. Mater. Med. 2008, 19, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Myung-Hyun, L.; Lee, D.Y.; Han, J.S. Mechanical properties, phase stability, and biocompatibility of (Y, Nb)-TZP/Al(2)O(3) composite abutments for dental implant. J. Biomed. Mater. Res. 2000, 53, 438–443. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsieh, C.-Y.; Huang, F.-H.; Cheng, L.-P. Preparation of zirconia loaded poly(acrylate) antistatic hard coatings on PMMA substrates. J. Appl. Polym. Sci. 2015, 132, 42411. [Google Scholar] [CrossRef]
- Sakthivel, M.; Franklin, D.; Guhanathan, S. pH-sensitive Itaconic acid based polymeric hydrogels for dye removal applications. Ecotoxicol. Environ. Saf. 2016, 134, 427–432. [Google Scholar] [CrossRef]
- Raghu, C.; Raghuveer, P. Itaconic acid Production—A short review. Int. J. Adv. Eng. Technol. Manag. Appl. Sci. 2017, 4, 8–15. [Google Scholar]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Kirimura, K.; Honda, Y.; Hattori, T. Gluconic and Itaconic Acids. Compr. Biotechnol. 2011, 143–147. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703. [Google Scholar] [CrossRef]
- Manicone, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Color Vision. Perspectives from Different Disciplines. Ethology 1999, 105, 184–185. [CrossRef]
- Krishnan, S.; Mohanty, S.; Nayak, S.K. An eco-friendly approach for toughening of polylactic acid from itaconic acid based elastomer. J. Polym. Res. 2017, 25. [Google Scholar] [CrossRef]
- Takahashi, M.; Osawa, S.; Jinnai, H.; Yamane, H.; Shiomi, H. Dispersion State of Zirconium Oxide Particles in Polymer Blends and Viscoelastic Behavior of the Composites. Nihon Reoroji Gakkaishi 2007, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mishra, T.; Kumar, A.; Verma, V.; Pandey, K.; Kumar, V. PEEK composites reinforced with zirconia nanofiller. Compos. Sci. Technol. 2012, 72, 1627–1631. [Google Scholar] [CrossRef]
- Kimble, L.D.; Bhattacharyya, D. In Vitro Degradation Effects on Strength, Stiffness, and Creep of PLLA/PBS: A Potential Stent Material. Int. J. Polym. Mater. 2014, 64, 299–310. [Google Scholar] [CrossRef]
- Fan, F.; Xia, Z.; Li, Q.; Li, Z.; Chen, H. ZrO2/PMMA Nanocomposites: Preparation and Its Dispersion in Polymer Matrix. Chin. J. Chem. Eng. 2013, 21, 113–120. [Google Scholar] [CrossRef]
- Gentili, P.L. The Fuzziness of the Molecular World and Its Perspectives. Molcules 2018, 23, 2074. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Itaconic-acid#section=UV-Spectra (accessed on 24 November 2020).
- Zagórski, Z.P. Diffuse reflection spectrophotometry (DRS) for recognition of products of radiolysis in polymers. Int. J. Polym. Mater. 2003, 52, 323–333. [Google Scholar] [CrossRef]
- Ciccòla, A.; Guiso, M.; Domenici, F.; Sciubba, F.; Bianco, A. Azo-pigments effect on UV degradation of contemporary art pictorial film: A FTIR-NMR combination study. Polym. Degrad. Stab. 2017, 140, 74–83. [Google Scholar] [CrossRef]


| Reference | bioPBS Matrix | IA | Colorant | ZrO2 |
|---|---|---|---|---|
| F1 | 100% | - | - | - |
| F2 | 96% | 4% | - | - |
| F3 | 90% | 10% | - | - |
| F4 | 96% | - | 4% | - |
| F5 | 98% | - | - | 2% |
| F6 | 96% | - | - | 4% |
| F7 | 94% | - | 4% | 2% |
| F8 | 92% | 4% | 4% | - |
| F9 | 86% | 10% | 4% | - |
| F10 | 94% | 4% | - | 2% |
| F11 | 88% | 10% | - | 2% |
| F12 | 90% | 4% | 4% | 2% |
| F13 | 84% | 10% | 4% | 2% |
| Material | Young′s Modulus (MPa) W0 | Young′s Modulus (MPa) W4 | Elongation at Break (%) W0 | Elongation at Break (%) W4 | Hardness (HRB) W0 | Hardness (HRB) W4 | WCA (°) W0 | WCA (°) W4 |
|---|---|---|---|---|---|---|---|---|
| F1 | 590 ± 30 | 685 ± 20 | 268 ± 27 | 262 ± 9 | 6.2 ± 1.3 | 5.1 ± 0.9 | 76 ± 1 | 69 ± 3 |
| F2 | 473 ± 37 | 866 ± 33 | 289 ± 119 | 1.5 ± 0.8 | 20.6 ± 2.6 | Break | 54 ± 3 | 54 ± 0 |
| F3 | 528 ± 49 | 953 ± 63 | 317 ± 75 | 0.67 ± 0.05 | 5.1 ± 0.4 | Break | 66 ± 3 | 47 ± 3 |
| F4 | 645 ± 24 | 740 ± 14 | 258 ± 16 | 60 ± 4 | 2.6 ± 1.4 | 2 ± 1.3 | 68 ± 3 | 70 ± 3 |
| F5 | 625 ± 13 | 740 ± 60 | 258 ± 7 | 173 ± 18 | 6.6 ± 1.1 | 1.6 ± 0.5 | 75 ± 3 | 66 ± 1 |
| F6 | 726 ± 31 | 960 ± 351 | 205 ± 68 | 192 ± 18 | 4.3 ± 1.3 | 1.1 ± 0.6 | 76 ± 3 | 71 ± 2 |
| F7 | 631 ± 31 | 739 ± 23 | 249 ± 16 | 225 ± 32 | 1.1 ± 0.8 | 1.1 ± 0.8 | 70 ± 3 | 66 ± 3 |
| F8 | 634 ± 59 | 966 ± 32 | 75 ± 65 | 0.88 ± 0.2 | 10.4 ± 1.5 | Break | 63 ± 4 | 56 ± 3 |
| F9 | 626 ± 52 | 946 ± 39 | 259 ± 133 | 0.27 ± 0.01 | 6.4 ± 0.7 | Break | 65 ± 3 | 58 ± 4 |
| F10 | 659 ± 52 | 1052 ± 67 | 247 ± 42 | 0.97 ± 0,4 | 17.4 ± 0.3 | Break | 59 ± 2 | 44 ± 3 |
| F11 | 587 ± 34 | 937 ± 24 | 279 ± 45 | 0.56 ± 0.28 | 5.0 ± 1.2 | Break | 67 ± 2 | 61 ± 3 |
| F12 | 610 ± 52 | 1027 ± 66 | 57 ± 7 | 0.88 ± 0.2 | 10.2 ± 1.6 | Break | 60 ± 3 | 61 ± 3 |
| F13 | 585 ± 103 | 979 ± 53 | 145 ± 108 | 0.3 ± 0.05 | 5.6 ± 0.6 | Break | 60 ± 4 | 59 ± 3 |
| Material Formulations | L* | a* | b* | ∆E* | Gloss (°) |
|---|---|---|---|---|---|
| White Control | 99.47 ± 0.00 | −0.08 ± 0.01 | −0.08 ± 0.01 | - | 121 ± 0 |
| F1–W0 | 85.80 ± 0.26 | −1.22 ± 0.03 | −1.04 ± 0.08 | 13.75 ± 0.26 | 78 ± 4 |
| F1–W4 | 86.05 ± 0.43 | −1.05 ± 0.03 | −1.60 ± 0.08 | 13.54 ± 0.43 | 72 ± 1 |
| F2–W0 | 86.84 ± 0.13 | −1.34 ± 0.04 | 3.53 ± 0.08 | 13.20 ± 0.13 | 72 ± 2 |
| F2–W4 | 87.34 ± 0.23 | −1.07 ± 0.02 | 3.02 ± 0.19 | 12.56 ± 0.25 | 53 ± 3 |
| F3–W0 | 84.01 ± 0.06 | −2.27 ± 0.02 | 9.92 ± 0.10 | 18.54 ± 0.06 | 63 ± 2 |
| F3–W4 | 84.37 ± 0.21 | −1.68 ± 0.14 | 8.08 ± 0.06 | 17.23 ± 0.23 | 17 ± 2 |
| F4–W0 | 35.09 ± 0.09 | 36.71 ± 0.26 | 16.06 ± 0.12 | 75.89 ± 0.08 | 77 ± 3 |
| F4–W4 | 34.84 ± 0.12 | 35.72 ± 0.17 | 15.39 ± 0.15 | 75.49 ± 0.18 | 77 ± 4 |
| F5–W0 | 86.43 ± 0.07 | −0.41 ± 0.03 | 8.97 ± 0.05 | 15.87 ± 0.04 | 70 ± 2 |
| F5–W4 | 86.50 ± 0.15 | −0.46 ± 0.01 | 8.41 ± 0.13 | 15.51 ± 0.06 | 71 ± 4 |
| F6–W0 | 88.34 ± 0.06 | 0.77 ± 0.03 | 13.29 ± 0.12 | 17.42 ± 0.09 | 68 ± 3 |
| F6–W4 | 88.41 ± 0.10 | 0.73 ± 0.05 | 13.07 ± 0.10 | 17.20 ± 0.11 | 75 ± 2 |
| F7–W0 | 39.30 ± 0.39 | 44.58 ± 0.12 | 12.51 ± 0.20 | 75.99 ± 0.30 | 66 ± 3 |
| F7–W4 | 39.01 ± 0.23 | 43.73 ± 0.17 | 11.60 ± 0.27 | 75.57 ± 0.32 | 60 ± 3 |
| F8–W0 | 36.18 ± 0.09 | 40.28 ± 0.65 | 15.7 ± 0.24 | 76.72 ± 0.38 | 66 ± 3 |
| F8–W4 | 36.04 ± 0.36 | 40.81 ± 0.99 | 15.43 ± 0.22 | 77.05 ± 0.40 | 8 ± 2 |
| F9–W0 | 34.88 ± 0.14 | 38.34 ± 0.38 | 15.39 ± 0.33 | 76.73 ± 0.20 | 66 ± 3 |
| F9–W4 | 34.78 ± 0.51 | 39.33 ± 1.03 | 15.38 ± 0.41 | 77.32 ± 1.03 | 11 ± 2 |
| F10–W0 | 87.62 ± 0.34 | −0.56 ± 0.04 | 11.55 ± 0.20 | 16.62 ± 0.25 | 71 ± 3 |
| F10–W4 | 88.05 ± 0.13 | −0.78 ± 0.04 | 10.83 ± 0.40 | 15.81 ± 0.33 | 26 ± 1 |
| F11–W0 | 85.44 ± 0.26 | −0.46 ± 0.02 | 11.38 ± 0.07 | 18.12 ± 0.19 | 61 ± 3 |
| F11–W4 | 85.82 ± 0.10 | −0.44 ± 0.05 | 10.56 ± 0.10 | 17.31 ± 0.13 | 29 ± 3 |
| F12–W0 | 38.25 ± 0.60 | 40.30 ± 0.13 | 12.70 ± 0.28 | 74.44 ± 0.52 | 63 ± 3 |
| F12–W4 | 37.84 ± 0.52 | 42.11 ± 0.46 | 12.83 ± 0.13 | 75.79 ± 0.42 | 5 ± 1 |
| F13–W0 | 38.88 ± 0.11 | 42.90 ± 0.11 | 12.98 ± 0.11 | 75.42 ± 0.11 | 67 ± 2 |
| F13–W4 | 38.89 ± 0.29 | 44.20 ± 0.21 | 13.03 ± 0.20 | 76.17 ± 0.34 | 8 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
G. Quiles, L.; Vidal, J.; Luzi, F.; Dominici, F.; Fernández Cuello, Á.; Castell, P. Color Fixation Strategies on Sustainable Poly-Butylene Succinate Using Biobased Itaconic Acid. Polymers 2021, 13, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010079
G. Quiles L, Vidal J, Luzi F, Dominici F, Fernández Cuello Á, Castell P. Color Fixation Strategies on Sustainable Poly-Butylene Succinate Using Biobased Itaconic Acid. Polymers. 2021; 13(1):79. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010079
Chicago/Turabian StyleG. Quiles, Lidia, Julio Vidal, Francesca Luzi, Franco Dominici, Ángel Fernández Cuello, and Pere Castell. 2021. "Color Fixation Strategies on Sustainable Poly-Butylene Succinate Using Biobased Itaconic Acid" Polymers 13, no. 1: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13010079