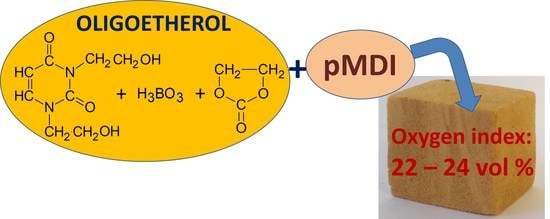
Hardly Flammable Polyurethane Foams with 1,3-Pyrimidine Ring and Boron Atoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 1,3-bis(2-hydroxyethyl)uracil
2.2. Oligoetherols Synthesis
2.2.1. Two-Step Synthesis of Oligoetherol
Reaction of BHEU with BA
Reactions of DOPDEDHB with EC
2.2.2. One-Pot Synthesis of Oligoetherol
2.3. Foams Synthesis
2.4. Analytical Methods
3. Results and Discussion
3.1. Oligoetherols Synthesis and Properties
3.2. Polyurethane Foams Synthesis
3.3. Properties of Foams
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2016. (In Polish) [Google Scholar]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Veronese, V.B.; Menger, R.K.; Forte, M.M.; Petzhold, C.L. Rigid polyurethane foam based on vegetable oil. J. Appl. Polym. Sci. 2011, 120, 530–537. [Google Scholar] [CrossRef]
- Gaidukov, S.; Maksimov, R.D.; Cabulis, U.; Plume, E.; Stunda-Zujeva, A. Mechanical properties of a rigid polyurethane/montmorillonite composite prepared by using a biopolyol. Mech. Compos. Mater. 2013, 49, 333–344. [Google Scholar] [CrossRef]
- Gaidukovs, S.; Kampars, V.; Bitenieks, J.; Bochkov, I.; Gaidukova, G.; Cabulis, U. Thermo-mechanical properties of polyurethane modified with graphite oxide and carbon nanotube particles. Integr. Ferroelectr. 2016, 173, 1–11. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y. Synthesis and Characterization of Polyols and Polyurethane Foams from PET Waste and Crude Glycerol. J. Polym. Environ. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Kuranska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Kuranska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguscik, M. Innovative porous polyurethane–polyisocyanurate foams based on rapeseed oil and modified with expandable graphite. Ind. Crops Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Gaidukova, G.; Ivdre, A.; Fridrihsone, A.; Verovkins, A.; Cabulis, U.; Gaidukovs, S. Polyurethane rigid foams obtained from polyols containing bio-based and recycled components and functional additives. Ind. Crops Prod. 2017, 102, 133–143. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czupryński, B. Questions of Chemistry and Technology of Polyurethanes; The Publishing House of the Academy of Bydgoszcz: Bydgoszcz, Poland, 2004. (In Polish) [Google Scholar]
- Sawicki, T. Plastics and fire hazard. Work Saf. 2003, 7–8, 43–45. (In Polish) [Google Scholar]
- Liu, X.; Hao, J.; Gaan, S. Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials. RSC Adv. 2016, 6, 74742–74756. [Google Scholar] [CrossRef] [Green Version]
- Kijowska, D.; Kucharski, M. Polyetherols from melamine and alkylene carbonates: Properties and application of foamed polyurethanes. J. Appl. Polym. Sci. 2004, 94, 2302–2308. [Google Scholar] [CrossRef]
- Lubczak, J.; Chmiel-Szukiewicz, E.; Duliban, J.; Głowacz-Czerwonka, D.; Lubczak, R.; Łukasiewicz, B.; Zarzyka, I.; Łodyga, A.; Tyński, P.; Minda-Data, D.; et al. Polyurethane foams with 1,3,5-triazine ring of improved thermal stability. Przem. Chem. 2014, 10, 1690–1697. (in Polish) [Google Scholar] [CrossRef]
- Cisek-Cicirko, I.; Lubczak, J. Polyurethane Foams of Improved Thermal Stability. Macromol. Mater. Eng. 2002, 287, 665–670. [Google Scholar] [CrossRef]
- Lubczak, J. Polyurethane foams with purine rings. Polimery 2007, 52, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Chmiel-Szukiewicz, E. Polyurethane foams with 1,3-pyrimidine ring. J. Appl. Polym. Sci. 2008, 109, 1708–1713. [Google Scholar] [CrossRef]
- Chmiel-Szukiewicz, E. Polyurethane foams based on polyetherols obtained from 6-aminouracil and alkylene carbonates. Polimery 2010, 55, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Chmiel-Szukiewicz, E. New foamed plastic based polyetherols obtained from 6-aminouracil, ethylene carbonate and propylene oxide. J. Appl. Polym. Sci. 2013, 127, 1595–1600. [Google Scholar] [CrossRef]
- Kania, E.; Lubczak, J. Polyurethane Foams with Pyrimidine Rings. Pol. J. Chem. Technol. 2014, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogenfree flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.R.; Tan, Y.; Huang, J.Q.; Wang, X.L.; Chen, L.; Wang, Y.Z. Inherently Flame-Retardant Flexible Polyurethane Foam with Low Content of Phosphorus-Containing Cross-Linking Agent. Ind. Eng. Chem. Res. 2014, 53, 1160–1171. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, Z.; Chen, Y.; Li, G.; Wang, L.; Tang, Y.; Pang, R.; Khan, Z.U.H.; Wan, P. Preparation and characterization of flame retardant polyurethane foams containing phosphorus–nitrogen-functionalized lignin. RSC Adv. 2014, 4, 55271–55279. [Google Scholar] [CrossRef]
- La Guardia, M.J.; Hale, R.C. Halogenated flame-retardant concentrations in settled dust, respirable and inhalable particulates and polyurethane foam at gymnastic training facilities and residences. Environ. Int. 2015, 79, 106–114. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-Sadowska, J.; Liszkowska, J. Modifications of the rigid polyurethane–polyisocyanurate foams. J. Appl. Polym. Sci. 2006, 100, 2020–2029. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B. New Compounds for Production of Polyurethane Foams. J. Appl. Polym. Sci. 2006, 102, 5918–5926. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. New polyol for production of rigid polyurethane-polyisocyanurate foams. Part 2: Preparation of rigid polyurethane-polyisocyanurate foams with the new polyol. J. Appl. Polym. Sci. 2010, 118, 2250–2256. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Boron-containing fire retardant rigid polyurethane-polyisocyanurate foams: Part II—Preparation and evaluation. J. Fire Sci. 2015, 33, 48–68. [Google Scholar] [CrossRef]
- Lubczak, J.; Łukasiewicz, B. Oligoetherols and polyurethane foams with 1,3,5-triazine ring and boron. Polimery 2012, 57, 819–829. [Google Scholar] [CrossRef]
- Lubczak, J.; Łukasiewicz, B.; Myśliwiec, B. Synthesis and applications of oligoetherols with perhydro-1,3,5-triazine ring and boron. J. Appl. Polym. Sci. 2013, 127, 2057–2066. [Google Scholar] [CrossRef]
- Chmiel, E.; Lubczak, J. Oligoetherols and polyurethane foams obtained from melamine diborate. J. Polym. Res. 2017, 24, 95. [Google Scholar] [CrossRef] [Green Version]
- Chmiel, E.; Lubczak, J.; Stagraczyński, R. Modification of polyurethane foams with 1,3,5-triazine ring and boron. Macromol. Res. 2017, 25, 317–324. [Google Scholar] [CrossRef]
- Chmiel-Szukiewicz, E. Method for Producing 1,3-bis(2-hydroxyethyl)uracil. Polish Patent 230024, 28 September 2018. [Google Scholar]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Gold nanoparticleenhanced target (AuNPET) as universal solution for laser desorption/ionization mass spectrometry analysis and imaging of low molecular weight compounds. Anal. Chim. Acta 2015, 875, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Cellular Plastics and Rubbers—Determination of Apparent (Bulk) Density; Polish (European) Standards PN-EN ISO 845:2010; Polish Committee for Standardization: Warsaw, Poland, 2010.
- Cellular Plastics, Rigid—Determination of Water Absorption; Polish (European) Standards PN-EN ISO 2896:1987; Polish Committee for Standardization: Warsaw, Poland, 1987.
- Cellular Plastics, Rigid—Test of Dimensional Stability; Polish (European) Standards PN-EN ISO 2796:1986; Polish Committee for Standardization: Warsaw, Poland, 1986.
- Thermal Insulation Products for Buildings—Factory Made Rigid Polyurethane Foam (PU) Products—Specification; Polish (European) Standards PN-EN 13165:2009; Polish Committee for Standardization: Warsaw, Poland, 2009.
- Cellular Plastics, Compression Test for Rigid Materials; Polish (European) Standards PN- EN ISO 844-1978; Polish Committee for Standardization: Warsaw, Poland, 1978.
- Cellular Plastics. Determination of Horizontal Burning Characteristics of Small Specimens Subjected to a Small Flame; European Standards ISO 9772:2012; Polish Committee for Standardization: Warsaw, Poland, 2012.
- Plastics—Determination of Burning Behaviour by Oxygen Index—Ambient-Temperature Test; Polish (European) Standards PN ISO 4589-2:1999; Polish Committee for Standardization: Warsaw, Poland, 1999.
- Flexible Cellular Polymeric Materials—Laboratory Characteristics of Small Specimens Subject to a Small Flame; Polish (European) Standards PN-EN ISO 3582-2002; Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)












| Oligoetherol—Product of Reaction | Composition Number | Composition * (g/100 g of Oligoetherol) | Foaming Process | Characteristics of Freshly Prepared Foams | ||||
|---|---|---|---|---|---|---|---|---|
| pMDI | TEA | Water | Time of Creaming (s) | Time of Expanding (s) | Time of Drying (s) | |||
| BHEU:BA:EC 1:2:12 | 1 | 128 | 2.16 | 2 | 52 | 10 | 1 | insufficiently grown, hard |
| 2 | 149 | 2.70 | 3 | 46 | 17 | 1 | small shrink, small regular pores, rigid | |
| BHEU:BA:EC 1:3:12 | 3 | 165 | 4.31 | 3 | 40 | 12 | 5 | rigid after time |
| 4 | 160 | 3.77 | 3 | 43 | 9 | 1 | rigid | |
| DOPDEDHB:EC 1:12 | 5 | 80 | 3.23 | 3 | 35 | 60 | 190 | unrigid |
| 6 | 120 | 4.86 | 3 | 25 | 43 | 1 | small shrink, small regular pores, rigid | |
| 7 | 140 | 3.85 | 3 | 32 | 20 | 1 | small regular pores, rigid | |
| DOPDEDHB:EC 1:16 | 8 | 120 | 4.31 | 3 | 79 | 23 | 1 | rigid after time |
| 9 | 140 | 3.50 | 3 | 27 | 11 | 1 | small regular pores, rigid | |
| No | Signal Position (M/z) | Probable Structure of Molecular Ion | Calc. Molecular Weight (g/mol) |
|---|---|---|---|
| 1 | 83.1 | Ethylene glycol + K+ − H2O | 83.0 |
| 2 | 89.1 | 1,4-dioxane + H+ | 89.1 |
| 3 | 233.1 | Tetraethylene glycol + K+ | 233.1 |
| 4 | 397.3 | DOPDEDHB + 2 OE − H2O + K+ DOPDEDHB + OE − H2O + CO2 + K+ | 397.1 397.1 |
| 5 | 415.1 | DOPDEDHB + 2 OE + K+ | 415.1 |
| 6 | 421.1 | DOPDEDHB + 3 OE + H+ DOPDEDHB + 2 OE + CO2 + H+ | 421.1 421.1 |
| 7 | 459.2 | DOPDEDHB + 3 OE + K+ DOPDEDHB + 2 OE + CO2+ K+ | 459.1 459.1 |
| 8 | 465.2 | DOPDEDHB + 4 OE + H+ DOPDEDHB + 3 OE + CO2+ H+ | 465.2 465.2 |
| 9 | 503.2 | DOPDEDHB + 4 OE + K+ DOPDEDHB + 3 OE + CO2+ K+ | 503.2 503.2 |
| 10 | 509.2 | DOPDEDHB + 5 OE + H+ DOPDEDHB + 4 OE + CO2+ H+ | 509.2 509.2 |
| 11 | 534.3 | DOPDEDHB + 6 OE − H2O DOPDEDHB + 5 OE − H2O + CO2 | 534.2 534.2 |
| 12 | 553.3 | DOPDEDHB + 6 OE + H+ DOPDEDHB + 5 OE + CO2+ H+ | 553.3 553.3 |
| 13 | 579. 4 | DOPDEDHB + 7 OE − H2O + H+ DOPDEDHB + 6 OE − H2O + CO2+ H+ | 579.3 579.3 |
| 14 | 597.3 | DOPDEDHB + 7 OE + H+ DOPDEDHB + 6 OE + CO2+ H+ | 597.3 597.3 |
| 15 | 641.2 | DOPDEDHB + 8 OE + H+ DOPDEDHB + 7 OE + CO2+ H+ | 641.3 641.3 |
| 16 | 773.5 | DOPDEDHB + 11 OE + H+ DOPDEDHB + 10 OE + CO2+ H+ | 773.4 773.4 |
| No | Signal Position (M/z) | Probable Structure of Molecular Ion | Calc. Molecular Weight (g/mol) |
|---|---|---|---|
| 1 | 83.1 | Ethylene glycol + K+ − H2O | 83.0 |
| 2 | 89.1 | 1,4-dioxane + H+ | 89.1 |
| 3 | 145.0 | BA + OE + K+ | 145.0 |
| 4 | 189.1 | BA + 2 OE + K+ | 189.0 |
| 5 | 227.1 | BHEU + OE − H2O + H+ | 227.1 |
| 6 | 233.1 | BA + 3 OE + K+ Tetraethylene glycol + K+ | 233.1 233.1 |
| 7 | 239.1 | BA + 4 OE + H+ | 239.1 |
| 8 | 245.1 | HEDOPEDHB + H+ BHEU + OE + H+ | 245.1 245.1 |
| 9 | 277.1 | BA + 3 OE − H2O + H+ BA + 4 OE + K+ | 277.1 277.1 |
| 10 | 283.2 | BHEU + OE + K+ BA + 5 OE + H+ | 283.1 283.2 |
| 11 | 289.2 | DOPDEDHB + H+ HEDOPEDHB + OE + H+ BHEU + 2 OE + H+ | 289.1 289.1 289.1 |
| 12 | 321.2 | BA + 5 OE + K+ | 321.1 |
| 13 | 327.2 | DOPDEDHB + K+ HEDOPEDHB + OE + K+ BA + 6 OE + H+ BHEU + 2 OE + K+ | 327.1 327.1 327.2 327.2 |
| 14 | 365.1 | BA + 6 OE + K+ | 365.1 |
| 15 | 371.2 | DOPDEDHB + OE + K+ HEDOPEDHB + 2 OE + K+ BA + 7 OE + H+ BHEU + 3 OE + K+ | 371.1 371.1 371.2 371.2 |
| 16 | 376.3 | DOPDEDHB + 2 OE BHEU + 4 OE | 376.0 376.3 |
| 17 | 415.1 | DOPDEDHB + 2 OE + K+ HEDOPEDHB + 3 OE + K+ BHEU + 4 OE + K+ | 415.1 415.1 415.1 |
| 18 | 421.2 | DOPDEDHB + 3 OE + H+ HEDOPEDHB + 4 OE + H+ BHEU + 5 OE + H+ | 421.1 421.2 421.2 |
| 19 | 465.2 | DOPDEDHB + 4 OE + H+ HEDOPEDHB + 5 OE + H+ BHEU + 6 OE + H+ | 465.2 465.2 465.2 |
| 20 | 553.2 | DOPDEDHB + 6 OE + H+ BHEU + 8 OE + H+ | 553.3 553.3 |
| 21 | 773.5 | DOPDEDHB + 11 OE + H+ BHEU + 13 OE + H+ | 773.4 773.4 |
| Oligoetherol—Product of Reaction | Viscosity (N × s/m2) × 103 | Density (g/cm3) | Surface Tension (N/m) × 103 |
|---|---|---|---|
| DOPDEDHB:EC 1:12 | 79,500 | 1.25 | 51 |
| DOPDEDHB:EC 1:16 | 192,600 | 1.24 | 52 |
| BHEU:BA:EC 1:2:12 | 10,000 | 1.24 | 50 |
| BHEU:BA:EC 1:3:12 | 7600 | 1.25 | 49 |
| Composition Number (From Oligoetherol) | Density (kg/m3) | Absorption of Water (vol%) | Linear Dimensions Stability at Temperature 150 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length Increase (%) | Width Increase (%) | Thickness Increase (%) | ||||||||
| After 5 Mmin | After 3 h | After 24 h | After 20 h | After 40 h | After 20 h | After 40 h | After 20 h | After 40 h | ||
| 2 (BHEU:BA:EC = 1:2:12) | 66 | 4.37 | 9.61 | 27.62 | 3.19 | 4.65 | 3.00 | 3.70 | 2.94 | 4.84 |
| 4 (BHEU:BA:EC = 1:3:12) | 91 | 4.44 | 11.22 | 18.21 | 0.70 | 0.95 | 0.28 | 0.56 | −0.29 | 0.16 |
| 7 (DOPDEDHB:EC = 1:12) | 72 | 3.76 | 7.76 | 18.40 | 2.47 | 3.40 | 1.51 | 2.15 | 4.21 | 5.24 |
| 9 (DOPDEDHB:EC = 1:16) | 56 | 2.21 | 5.00 | 8.05 | 0.78 | 2.33 | 0.72 | 2.77 | −1.96 | 1.19 |
| AU:EC = 1:6 [19] | 32 | 4.45 | 6.4 | 8.7 | 0.17 | 0.34 | −0.61 | −0.31 | 2.23 | 2.68 |
| AU:EC:PO = 1:6:6 [20] | 42 | 2.57 | 4.15 | 5.28 | −0.29 | 2.03 | 2.36 | 4.72 | −1.94 | 0 |
| Composition Number (From Oligoetherol) | The Mass Loss After 30 Days Heating at Temperature (wt %) | Thermal Analysis | ||||
|---|---|---|---|---|---|---|
| 150 °C | 175 °C | 200 °C | T10% (°C) | T25% (°C) | T50% (°C) | |
| 2 (BHEU:BA:EC = 1:2:12) | 19 | 35 | - | 235 | 252 | 303 |
| 4 (BHEU:BA:EC = 1:3:12) | 24 | 38 | - | 202 | 242 | 299 |
| 7 (DOPDEDHB:EC = 1:12) | 16 | 34 | - | 228 | 269 | 322 |
| 9 (DOPDEDHB:EC = 1:16) | 19 | 34 | - | 230 | 255 | 302 |
| AU:EC = 1:6 [19] | 7 | 23 | 33 | 260 | 320 | 550 |
| AU:EC:PO = 1:6:6 [20] | 13 | 29 | 41 | 235 | 280 | 330 |
| Composition Number (From Oligoetherol) | Thermal Conductivity Coefficient Λ [W/m × K] | Compression Strength | ||
|---|---|---|---|---|
| Before Exposition | After Exposition at 150 °C | |||
| σM (MPa) | εM (%) | σ10 (MPa) | ||
| 2 (BHEU:BA:EC = 1:2:12) | 0.0357 | 0.33 | 7.08 | 0.58 |
| 4 (BHEU:BA:EC = 1:3:12) | 0.0368 | 0.30 | 7.99 | 0.81 |
| 7 (DOPDEDHB:EC = 1:12) | 0.0357 | 0.29 | 8.33 | 1.12 |
| 9 (DOPDEDHB:EC = 1:16) | 0.0349 | 0.32 | 9.08 | 1.27 |
| Composition Number (From Oligoetherol) | Horizontal Burning Tests | Oxygen Index (vol%) | ||
|---|---|---|---|---|
| Linear Burning Rate (mm/s) | Distance Burnt (mm) | Mass Loss During Burning (% mas.) | ||
| 2 (BHEU:BA:EC = 1:2:12) | 3.42 | 18 | 4.35 | 22.3 |
| 4 (BHEU:BA:EC = 1:3:12) | 2.06 | 11 | 3.81 | 24.1 |
| 7 (DOPDEDHB:EC = 1:12) | 2.05 | 13 | 3.84 | 22.1 |
| 9 (DOPDEDHB:EC = 1:16) | 2.68 | 20 | 4.97 | 22.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel-Szukiewicz, E. Hardly Flammable Polyurethane Foams with 1,3-Pyrimidine Ring and Boron Atoms. Polymers 2021, 13, 1603. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101603
Chmiel-Szukiewicz E. Hardly Flammable Polyurethane Foams with 1,3-Pyrimidine Ring and Boron Atoms. Polymers. 2021; 13(10):1603. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101603
Chicago/Turabian StyleChmiel-Szukiewicz, Elżbieta. 2021. "Hardly Flammable Polyurethane Foams with 1,3-Pyrimidine Ring and Boron Atoms" Polymers 13, no. 10: 1603. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101603