Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of NR Compounds
3. Results and Discussion
3.1. Thermal Stability of Fillers and Ionic Liquids
3.2. Effect of Fillers and Ionic Liquids on Cure Characteristics of NR Compounds and Crosslink Density of Vulcanizates
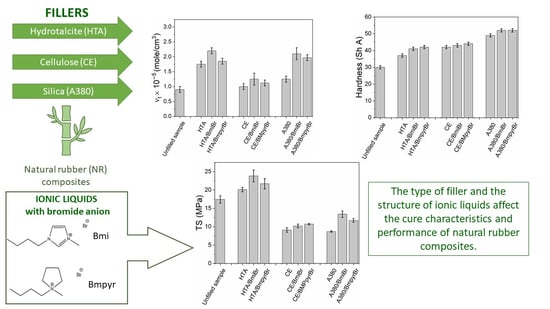
3.3. Effect of Fillers and Ionic Liquids on Tensile Properties of NR Vulcanizates
3.4. Effect of Fillers and Ionic Liquids on Mechanical Properties of NR Vulcanizates under Dynamic Conditions
3.5. Effect of Fillers and Ionic Liquids on Thermal Stability of NR Vulcanizates
3.6. FT-IR Analysis of NR Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Wang, Y.; Xu, W.; Shi, B. Natural Rubber-Based Elastomer Reinforced by Chemically Modified Multiscale Leather Collagen Fibers with Excellent Toughness. ACS Sustain. Chem. Eng. 2020, 8, 5091–5099. [Google Scholar] [CrossRef]
- Bokobza, L. The Reinforcement of Elastomeric Networks by Fillers. Macromol. Mater. Eng. 2004, 289, 607–621. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowińska, A. Thermal Characterization of the Effect of Fillers and Ionic Liquids on the Vulcanization and Properties of Acrylonitrile–Butadiene Elastomer. J. Therm. Anal. Calorim. 2019, 138, 4359–4373. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadyay, R.; De, S.K. Effect of Vulcanization Temperature and Different Fillers on the Properties of Efficiently Vulcanized Natural Rubber. Rubber Chem. Technol. 1979, 52, 263–277. [Google Scholar] [CrossRef]
- Da Costa, H.M.; Visconte, L.L.Y.; Nunes, R.C.R.; Furtado, C.R.G. Rice-Husk-Ash-Filled Natural Rubber. II. Partial Replacement of Commercial Fillers and the Effect on the Vulcanization Process. J. Appl. Polym. Sci. 2003, 87, 1405–1413. [Google Scholar] [CrossRef]
- Nygil, T.; Rajamathi, M. Intracrystalline Oxidation of Thiosulfate-Intercalated Layered Double Hydroxides. Langmuir 2009, 25, 2212–2216. [Google Scholar] [CrossRef]
- Costa, F.R.; Saphiannikova, M.; Wagenknecht, U.; Heinrich, G. Layered Double Hydroxide Based Polymer Nanocomposites. Adv. Polym. Sci. 2007, 210, 101–168. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Leroux, F.; Besse, J.P. Polymer Interleaved Layered Double Hydroxide: A New Emerging Class of Nanocomposites. Chem. Mater. 2001, 13, 3507–3515. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Stockelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in Layered Double Hydroxide (LDH)—Based Elastomer Composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Pradhan, S.; Costa, F.R.; Wagenknecht, U.; Jehnichen, D.; Bhowmick, A.K.; Heinrich, G. Elastomer/LDH Nanocomposites: Synthesis and Studies on Nanoparticle Dispersion Mechanical Properties and Interfacial Adhesion. Eur. Polym. J. 2008, 44, 3122–3132. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Das, A.; Leuteritz, A.; Mahaling, R.N.; Wagenknecht, U.; Heinrich, G. Structural Characteristics and Flammability of Fire Reatrding EPDM/Layered Double Hydroxide (LDH) Nanocomposites. RSC Adv. 2012, 2, 3927–3933. [Google Scholar] [CrossRef]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites Based on Chloroprene Rubber: Effect of Chemical Nature and Organic Modification of Nanoclay on the Vulcanizate Properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- He, X.; Li, T.; Shi, Z.; Wang, X.; Xue, F.; Wu, Z.; Chen, Q. Thermal-Oxidative Aging Behavior of Nitrile-Butadiene Rubber/Functional LDHs Composites. Polym. Degrad. Stabil. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Xiao, S.; Tan, Y.; Xu, J.; Xiong, C.; Wang, X.; Su, S. Lignosulfonate as Dispersant for Layered Double Hydroxide in Nitrile–Butadiene Rubber Composites. Appl. Clay Sci. 2014, 97, 91–95. [Google Scholar] [CrossRef]
- Laskowska, A.; Zaborski, M.; Boiteux, G.; Gain, O.; Marzec, A.; Maniukiewicz, W. Ionic Elastomers Based on Carboxylated Nitrile Rubber (XNBR) and Magnesium Aluminum Layered Double Hydroxide (hydrotalcite). eXPRESS Polym. Lett. 2014, 8, 374–386. [Google Scholar] [CrossRef]
- Lipinska, M.; Gaca, M.; Zaborski, M. Curing Kinetics and Ionic Interactions in Layered Double Hydroxides–Nitrile Rubber Mg–Al-LDHs–XNBR Composites. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Pradhan, B.; Srivastava, S.K.; Ananthakrishan, R.; Saxena, A. Preparation and Characterization of Exfoliated Layered Double Hydroxide/Silicone Rubber Nanocomposites. J. Appl. Polym. Sci. 2011, 119, 343–351. [Google Scholar] [CrossRef]
- Pradhan, B.; Srivastava, S.K.; Bhowmick, A.K.; Saxena, A. Effect of Bilayered Stearate Ion-Modified Mg-Al Layered Double Hydroxide on the Thermal and Mechanical Properties of Silicone Rubber Nanocomposites. Polym. Int. 2012, 61, 458–465. [Google Scholar] [CrossRef]
- Kotal, M.; Srivastava, S.K. Structure-Property Relationship of Polyurethane/Modified Magnesium Aluminium Layered Double Hydroxide Nanocomposites. Int. J. Plast. Technol. 2011, 15, 61–68. [Google Scholar] [CrossRef]
- Das, A.; George, J.J.; Kutlu, B.; Leuteritz, A.; Wang, D.-Y.; Rooi, S.; Jurk, R.; Rajeshbabu, R.; Stockelhuber, K.W.; Galiatsatos, V.; et al. A Novel Thermotropic Elastomer Based on Highly-Filled LDH-SSB Composites. Macromol. Rapid Commun. 2012, 33, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Macedo da Silva, V.; Reis Nunes, R.C.; Furtado de Sousa, A.M. Epoxidized Natural Rubber and Hydrotalcite Compounds: Rheological and Thermal Characterization. Polimeros 2017, 27, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Wang, D.-Y.; Leuteritz, A.; Subramaniam, K.; Greenwell, C.; Wagenknecht, U.; Heinrich, G. Preparation of Zinc Oxide Free, Transparent Rubber Nanocomposites Using a Layered Double Hydroxide Filler. J. Mater. Chem. 2011, 21, 7194–7200. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Iulianelli, G.C.V.; Tavares, M.I.B. The Use of Cellulose Nanofillers in Obtaining Polymer Nanocomposites: Properties, Processing and Applications. Mater. Sci. Appl. 2016, 7, 257–295. [Google Scholar] [CrossRef] [Green Version]
- Hubbe, M.A.; Rojas, O.J.; Lucia, L.A.; Sain, M. Cellulosic Nanocomposites: A Review. BioResources 2008, 3, 929–980. [Google Scholar]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Effects of Ionic Liquid on Cellulosic Nanofiller Filled Natural Rubber Bionanocomposites. J. Colloid Interface Sci. 2021, 591, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Laka, M.; Chernvavskaya, S.; Maskavs, M. Cellulose-Containing Fillers for Polymer Composites. Mech. Compos. Mater. 2003, 39, 183–188. [Google Scholar] [CrossRef]
- Spanic, N.; Jambrekovic, V.; Sernek, M.; Medved, S. Influence of Natural Fillers on Thermal and Mechanical Properties and Surface Morphology of Cellulose Acetate-Based Biocomposites. Int. J. Polym. Sci. 2019, 2019, 065024. [Google Scholar] [CrossRef]
- Santos, F.A.; Tavares, M.I.B. Development of Biopolymer/Cellulose/Silica Nanostructured Hybrid Materials and Their Characterization by NMR Relaxometry. Polym. Test. 2015, 47, 92–100. [Google Scholar] [CrossRef]
- AlMaadeed, M.A.; Nogellova, Z.; Micusik, M.; Novak, I.; Krupa, I. Mechanical Sorption and Adhesive Properties of Composites Based on Low Density Polyethylene Filled with Date Palm Wood Powder. Mater. Des. 2014, 53, 29–37. [Google Scholar] [CrossRef]
- Ni’mah, H.; Ningrum, E.O.; Nur Rizkivah, S.D.; Gede Chandra Divta, I.G.A.; Subaghio, M.M.A. Effect of Particle Size and Crystallinity of Cellulose Filler on the Properties of Poly (L-Lactic Acid): Mechanical Property and Thermal Stability. AIP Conf. Proc. 2017, 1840, 0900009. [Google Scholar] [CrossRef]
- Olsson, C.; Idstrom, A.; Nordstiema, L.; Westman, G. Influence of Water on Swelling and Dissolution of Cellulose in 1-Ethyl-3-Methylimidazolium Acetate. Carbohydr. Polym. 2014, 99, 438–446. [Google Scholar] [CrossRef]
- Sporl, J.M.; Batti, F.; Vocht, M.-P.; Raab, R.; Muller, A.; Hermanutz, F.; Buchmeiser, M.R. Ionic Liquid Approach Toward Manufacture and Full Recycling of All-Cellulose Composites. Macromol. Mater. Eng. 2018, 303, 1700335. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Tang, L.; Gao, X.; Zhang, W.; Ma, J.; Zhang, L. Solvent Regulation Approach for Preparing Cellulose-Nanocrystal-Reinforced Regenerated Cellulose Fibers and Their Properties. ACS Omega 2019, 4, 2001–2008. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Tang, Z.; Zhu, B.; GuO, L.; Jia, D. Functional Thiol Ionic Liquids as Novel Interfacial Modifiers in SBR/HNTs Composites. Polymer 2011, 52, 1337–1344. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Hu, Y.; Ding, Y. Piezoresistive Properties of Nanocomposites Based on Silicone Rubber and Ionic Liquid-Functionalized Carbon Black. Mater. Lett. 2016, 182, 218–222. [Google Scholar] [CrossRef]
- Das, A.; Stockelhuber, K.W.; Jurk, R.; Fritzsche, J.; Kluppel, M.; Heinrich, G. Coupling Activity of Ionic Liquids Between Diene Elastomers and Multi-walled Carbon Nanotubes. Carbon 2009, 47, 3313–3321. [Google Scholar] [CrossRef]
- Hussain, M.; Yasin, S.; Akram, A.; Xu, H.; Song, Y.; Zheng, Q. Influence of Ionic Liquids on the Structure and Rheological Behaviors of Silica-Filled Butadiene Rubber. Ind. Chem. Res. 2019, 58, 18205–18212. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H.; Ding, L.; Xiong, X.; Gong, X. The Mechanism of Carbon-Silica Dual Phase Filler Modified by Ionic Liquid and Its Reinforcing on Natural Rubber. Polym. Comp. 2015, 36, 1721–1730. [Google Scholar] [CrossRef]
- Sattayanurak, S.; Noordermeer, J.W.M.; Sahakaro, K.; Kaewsakul, W.; Dierkes, W.K.; Blume, A. Silica-Reinforced Natural Rubber: Synergistic Effects by Addition of Small Amounts of Secondary Fillers to Silica-Reinforced Natural Rubber Tire Tread Compounds. Adv. Mater. Sci. Eng. 2019, 2019, 5891051. [Google Scholar] [CrossRef] [Green Version]
- Medalia, A.I.; Kraus, G. Reinforcement of Elastomers by Particulate Fillers. In Science and Technology of Rubber, 2nd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Elsevier Academic Press: London, UK, 2005; p. 423. [Google Scholar]
- Noriman, N.Z.; Ismail, H. Properties of Styrene Butadiene Rubber (SBR)/Recycled Acrylonitrile Butadiene Rubber (NBRr) Blends: The Effects of Carbon Black/Silica (CB/Sil) Hybrid Filler and Silane Coupling Agent, Si69. J. Appl. Polym. Sci. 2011, 124, 19–27. [Google Scholar] [CrossRef]
- Phewphong, P.; Sae-oui, P.; Sirisinha, C. The Use of Dynamic Mechanical Thermal Analysis Technique for Determining an Uneven Distribution of Precipitated Silica in CPE/NR Blends. Polym. Test. 2008, 27, 873–880. [Google Scholar] [CrossRef]
- Kosmalska, A.; Zaborski, M.; Slusarski, L. Adsorption of Curatives and Activity of Silica Towards Elastomers. Macromol. Symp. 2003, 194, 269–275. [Google Scholar] [CrossRef]
- Maciejewska, M.; Siwek, M. The Influence of Curing Systems on the Cure Characteristics and Physical Properties of Styrene–Butadiene Elastomer. Materials 2020, 13, 5329. [Google Scholar] [CrossRef]
- Bokobza, L.; Rapoport, O. Reinforcement of Natural Rubber. J. Appl. Polym. Sci. 2002, 85, 2301–2316. [Google Scholar] [CrossRef]
- Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- Mathew, G.; Huh, M.-Y.; Rhee, J.M.; Lee, M.-H.; Nah, C. Improvement of Properties of Silica-Filled Styrene-Butadiene Rubber Composites Through Plasma Surface Modification of Silica. Polym. Adv. Technol. 2004, 15, 400–408. [Google Scholar] [CrossRef]
- Castellano, M.; Conzatti, L.; Costa, G.; Falqui, L.; Turturro, A.; Valenti, B.; Negroni, F. Surface Modification of Silica: 1. Thermodynamic Aspects and Effect on Elastomer Reinforcement. Polymer 2005, 46, 695–703. [Google Scholar] [CrossRef]
- Castellano, M.; Conzatti, L.; Turturro, A.; Costa, G.; Busca, G. Influence of the Silane Modifiers on the Surface Thermodynamic Characteristics and Dispersion of the Silica into Elastomer Compounds. J. Phys. Chem. B 2007, 111, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Aso, O.; Eguiazábal, J.I.; Nazábal, J. The Influence of Surface Modification on the Structure and Properties of a Nanosilica Filled Thermoplastic Elastomer. Compos. Sci. Technol. 2007, 67, 2854–2863. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Thermal Analysis and Mechanical Methods Applied to Studying Properties of SBR Compounds Containing Ionic Liquids. Polym. Test. 2017, 61, 349–363. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Zheng, Q.; Song, Y. Influence of Ionic Liquids on Rheological Behaviors of Polyisoprene Rubber/Silica Compounds. Polymer 2019, 183, 121898. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, X.; Jia, H.; Wang, J.; Ji, Q.; Xu, Z. Influence of Ionic Liquid on The Polymer–Filler Coupling and Mechanical Properties of Nano-Silica Filled Elastomer. J. Appl. Polym. Sci. 2017, 134, 44478. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, Q.; Zhang, Y.; Liu, F.; Han, J.; Wu, C. High-Performance Natural Rubber Latex Composites Developed by a Green Approach Using Ionic Liquid-Modified Multiwalled Carbon Nanotubes. J. Appl. Polym. Sci. 2018, 135, 46588. [Google Scholar] [CrossRef]
- Sowińska, A.; Maciejewska, M.; Grajewska, A. Bis(trifluoromethylsulfonyl)imide Ionic Liquids Applied for Fine-Tuning the Cure Characteristics and Performance of Natural Rubber Composites. Int. J. Mol. Sci. 2021, 22, 3678. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Das, A.; Basak, S.; Tahir, M.; Wießner, S.; Fischer, D.; Reuter, U.; Stöckelhuber, K.W.; Bhowmick, A.K.; Do, Q.K.; et al. Effect of Different Ionic Liquids on the Dispersion and Phase Selective Wetting of Carbon Nanotubes in Rubber Blends. Polymer 2016, 105, 284–297. [Google Scholar] [CrossRef]
- Krainoi, A.; Kummerlöwe, C.; Nakaramontri, Y.; Wisunthorn, S.; Vennemann, N.; Pichaiyut, S.; Kiatkamjornwong, S.; Nakason, C. Influence of Carbon Nanotube and Ionic Liquid on Properties of Natural Rubber Nanocomposites. eXPRESS Polym. Lett. 2019, 13, 327–348. [Google Scholar] [CrossRef]
- Hong, S.H.; Tung, T.T.; Huyen Trang, L.K.; Kim, T.J.; Suh, K.S. Preparation of Single-Walled Carbon Nanotube (SWNT) Gel Composites Using Poly (Ionic Liquids). Colloid. Polym. Sci. 2010, 288, 1013–1018. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 6502-3:2018, Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Rheometer; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- International Organization for Standardization. ISO 11357-1:2016, Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- International Organization for Standardization. ISO 1817:2015, Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Properties of Chemically Modified (Selected Silanes) Lignocellulosic Filler and Its Application in Natural Rubber Biocomposites. Materials 2020, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 37:2017, Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, Electrochemical and Radiolytic Stabilities of Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef] [PubMed]
- Feder-Kubis, J.; Zabielska-Matejuk, J.; Stangierska, A.; Przybylski, P.; Jacqemin, J.; Geppert-Rybczynska, M. Toward Designing “Sweet” Ionic Liquids Containing Natural Terprene Moiety as Effective Wood Preservatives. ACS Sustain. Chem. Eng. 2019, 7, 15628–15639. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Liu, Y.; Zhang, S.; Skov, A.L. Screening of Ionic Liquids for Keratin Dissolution by Means of COSMO-RS and Experimental Verification. ACS. Sustain. Chem. Eng. 2018, 6, 17314–17322. [Google Scholar] [CrossRef]
- Awad, W.H.; Gilman, J.W.; Nyden, M.; Harris, R.H.; Sutto, T.E.; Callahan, J.; Trulove, P.C.; DeLong, H.C.; Fox, D.M. Thermal Degradation Studies of Alkyl−imidazolium Salts and Their Application in Nanocomposites. Thermochim. Acta 2004, 409, 3–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, Q.; Cheng, H.; Frost, R.L. Thermal Stability of Styrene Butadiene Rubber (SBR) Composites Filled with Kaolinite/Silica Hybrid Filler. J. Therm. Anal. Calorim. 2014, 115, 1013–1020. [Google Scholar] [CrossRef]
- Scheirs, J.; Camino, G.; Tumiatti, W. Overview of Water Evolution During the Thermal Degradation of Cellulose. Eur. Polym. J. 2001, 37, 933–942. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Banyasz, J.L.; Li, S.; Lyons-Hart, J.L.; Shafer, K.H. Cellulose Pyrolysis: The Kinetics of Hydroxyacetaldehyde Evolution. J. Anal. Appl. Pyrol. 2001, 57, 223–248. [Google Scholar] [CrossRef]
- Banyasz, J.L.; Li, S.; Lyons-Hart, J.; Shafer, K.H. Gas Evolution and the Mechanism of Cellulose Pyrolysis. Fuel 2001, 80, 1757–1763. [Google Scholar] [CrossRef]
- Radlein, D.; Grinshpun, A.; Piskorz, J.; Scott, D.S. On the Presence of Anhydro-Oligosaccharides in the Sirups from the Fast Pyrolysis of Cellulose. J. Anal. Appl. Pyrol. 1987, 12, 39–49. [Google Scholar] [CrossRef]
- Arisz, P.W.; Lomax, J.A.; Boon, J.J. High-Performance Liquid Chromatography/Chemical Ionization Mass Spectrometric Analysis of Pyrolysates of Amylose and Cellulose. Anal. Chem. 1990, 62, 1519–1522. [Google Scholar] [CrossRef]
- Mamleev, V.; Bourbigot, S.; Yvon, J. Kinetic Analysis of the Thermal Decomposition of Cellulose: The Main Step of Mass Loss. J. Anal. Appl. Pyrol. 2007, 80, 151–165. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In Situ Investigation of the Thermal Decomposition of Co-Al Hydrotalcite in Different Atmospheres. J. Mater. Chem. 2011, 11, 821–830. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Ionic Liquids as Coagents for Sulfur Vulcanization of Butadiene–Styrene Elastomer Filled with Carbon Black. Polym. Bull. 2018, 75, 4499–4514. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, L. Natural Rubber Nanocomposites: A Review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.D.; Tang, Z.H.; Guo, B.C.; Zhu, L.X.; Jia, D.M. Synthesis of Novel Functional Liquid and its Application as a Modifier in SBR/Silica Composites. eXPRESS Polym. Lett. 2010, 4, 692–703. [Google Scholar] [CrossRef]
- Lipinska, M.; Soszka, K. Viscoelastic Behavior, Curing and Reinforcement Mechanism of Various Silica and POSS Filled Methyl-Vinyl Polysiloxane MVQ Rubber. Silicon 2019, 11, 2293–2305. [Google Scholar] [CrossRef] [Green Version]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. The Potential Application of Cereal Straw as a Bio-filler for Elastomer Composites. Polym. Bull. 2019, 77, 2021–2038. [Google Scholar] [CrossRef] [Green Version]
- Heideman, G.; Noordermeer, J.W.M.; Datta, R.N.; van Baarle, B. Multifunctional Additives as Zinc-Free Curatives for Sulfur Vulcanization. Rubber Chem. Technol. 2006, 79, 561–588. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M. Effect of Ionic Liquids and Surfactants on Zinc Oxide Nanoparticle Activity in Crosslinking of Acrylonitrile Butadiene Elastomer. J. Appl. Polym. Sci. 2010, 116, 155–164. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of Natural Rubber with Silica/Carbon Black Hybrid Filler. Polym Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Joy, J.; George, E.; Thomas, S.; Anas, S. Effect of Filler Loading on Polymer Chain Confinement and Thermomechanical Properties of Epoxy/Boron Nitride (h-BN) Nanocomposites. New J. Chem. 2020, 44, 4494–4503. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wei, W.L.; Hsu, K.Y.; Ho, W.H. Thermal Stability of Epoxy-Silica Hybrid Materials by Thermogravimetric Analysis. Thermochim. Acta 2004, 412, 139–147. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsu, C.Y.; Wei, W.L.; Jeng, R.J. Preparation and Thermal Properties of Epoxy-Silica Nanocomposites from Nanoscale Colloidal Silica. Polymer 2003, 44, 5159–5167. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of Silica Content on Thermal Stability of Fumed Silica/Epoxy Composites. Polym. Degrad. Stabil. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Wu, W.; Li, W.; Han, B.; Zhang, Z.; Jiang, T.; Liu, Z. A Green and Effective Method to Synthesize Ionic Liquids: Supercritical CO2 Route. Green Chem. 2005, 7, 701–705. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rao, G.R. Synthesis and Characterization of Hybrid Molecular Material Prepared by Ionic Liquid and Silicotungstic Acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; Wang, Y.; Li, H. Prediction of the Solvation and Structural Properties of Ionic Liquids in Water by Two-Dimensional Correlation Spectroscopy. J. Phys. Chem. B. 2008, 112, 6411–6419. [Google Scholar] [CrossRef]
- Roth, C.; Chatzipapadopoulos, S.; Kerle, D.; Friedriszik, F.; Lutgens, M.; Lochbrunner, S.; Kuhn, O.; Ludwig, R. Hydrogen Bonding in Ionic Liquids Probed by Linear and Nonlinear Vibrational Spectroscopy. New J. Phys. 2012, 14, 105026–105042. [Google Scholar] [CrossRef] [Green Version]
- Katsyuba, S.A.; Vener, M.V.; Zvereva, E.E.; Fei, Z.; Scopelliti, R.; Laurenczy, G.; Yan, N.; Paunescu, E.; Dyson, P.J. How Strong Is Hydrogen Bonding in Ionic Liquids? Combined X-Ray Crystallographic, Infrared/Raman Spectroscopic, and Density Functional Theory Study. J. Phys. Chem. B. 2013, 117, 9094–9105. [Google Scholar] [CrossRef]
- Kotov, N.; Sturcova, A.; Zhigunov, A.; Raus, V.; Dybal, J. Structural Transitions of 1-Butyl-3-methylimidazolium Chloride/Water Mixtures Studied by Raman and FTIR Spectroscopy and WAXS. Cryst. Growth Des. 2016, 16, 1958–1967. [Google Scholar] [CrossRef]
- Chalid, M.; Husnil, Y.A.; Puspitasari, S.; Cifriadi, A. Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers 2020, 12, 3017. [Google Scholar] [CrossRef]
- Aielo, P.B.; Borges, F.A.; Romeira, K.M.; Miranda, M.C.R.; de Arruda, L.B.; Filho, P.N.L.; Drago, B.C.; Herculano, R.D. Evaluation of Sodium Diclofenac Release Using Natural Rubber Latex as Carrier. Mater. Res. 2014, 17, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Benicio, L.P.F.; Silva, R.A.; Lopes, J.A.; Eulalio, D.; dos Santos, R.M.M.; de Aguino, L.A.; Vergutz, L.; Novais, R.F.; da Costa, L.M.; Pinto, F.G.; et al. Layered Double Hydroxides: Nanomaterials for Applications in Agriculture. Rev. Bras. Cienc. Solo. 2015, 39, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Hussein, M.Z.B.; Oksman, K. Preparation of Cellulose Nanofibers with Hydrophobic Surface Characteristics. Cellulose 2010, 17, 299–307. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR Study on Cellulose with the Varied Moisture Contents: Insight into the Supramolecular Structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.I.; Alvarez, V.A.; Cyras, V.P.; Vazquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Agrebi, F.; Ghorbel, N.; Bresson, S.; Abbas, O.; Kallel, A. Study of Nanocomposites Based on Cellulose Nanoparticles and Natural Rubber Latex by ATR/FTIR Spectroscopy: The Impact of Reinforcement. Polym. Compos. 2019, 40, 2076–2087. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational Spectroscopy of Selective Dental Restorative Materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]














| Name | Abbreviation | CAS Number | Purity (%) | Water Content (wt.%) |
|---|---|---|---|---|
| 1-butyl-3-methylimidazolium bromide | BmiBr | 85100-77-2 | ≥99.0% | <0.5% |
| 1-butyl-1-methylpyrrolidinium bromide | BmpyrBr | 93457-69-3 | ≥99.0% | ≤0.5% |
| Ingredient, Phr | Unfilled Sample (R0) | NR/A380 (R1) | NR/HTA (R2) | NR/CE (R3) |
|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 |
| Sulfur | 2 | 2 | 2 | 2 |
| ZnO | 5 | 5 | 5 | 5 |
| MBT | 2 | 2 | 2 | 2 |
| Stearin | 1 | 1 | 1 | 1 |
| Silica | ₋ | 30 | ₋ | ₋ |
| Hydrotalcite | ₋ | ₋ | 30 | ₋ |
| Cellulose | ₋ | ₋ | ₋ | 30 |
| Ingredient, Phr | NR/BmiBr (IL1–IL3) | NR/BmpyrBr (IL4–IL6) |
|---|---|---|
| NR | 100 | 100 |
| Sulfur | 2 | 2 |
| ZnO | 5 | 5 |
| MBT | 2 | 2 |
| Stearin | 1 | 1 |
| Filler * | 30 | 30 |
| BmiBr | 3 | ₋ |
| BmpyrBr | ₋ | 3 |
| Filler | TDTG (°C) | Mass Losses (%) |
|---|---|---|
| Hydrotalcite | 240 | Δm25–250 °C: 13.9 |
| 445 | ∆m250–800 °C: 31.3 | |
| Cellulose | 70 | ∆m25–150 °C: 10.1 |
| 365 | ∆m150–500 °C: 86.5 | |
| 630 | ∆m550–800 °C: 3.8 | |
| Silica A380 | 70 | ∆m25–800 °C: 6.4 |
| Compounds | Smin (MPa) | Smax (MPa) | ∆S (MPa) | t02 (min) | t90 (min) |
|---|---|---|---|---|---|
| Unfilled sample | 0.3 | 5.3 | 5.0 | 0.9 | 2.2 |
| BmiBr | 0.2 | 6.0 | 6.2 | 0.7 | 1.7 |
| BmpyrBr | 0.2 | 5.6 | 5.8 | 0.8 | 1.9 |
| Hydrotalcite | |||||
| HTA | 0.2 | 7.5 | 7.3 | 0.7 | 1.8 |
| HTA/BmiBr | 0.2 | 9.1 | 8.9 | 0.6 | 1.6 |
| HTA/BmpyrBr | 0.2 | 8.1 | 7.9 | 0.6 | 2.1 |
| Cellulose | |||||
| CE | 0.2 | 7.6 | 7.4 | 0.8 | 2.2 |
| CE/BmiBr | 0.2 | 8.3 | 8.1 | 0.7 | 2.2 |
| CE/BmpyrBr | 0.2 | 7.3 | 7.1 | 0.7 | 2.4 |
| Silica A380 | |||||
| A380 | 7.7 | 12.9 | 5.2 | 3.9 | 15.9 |
| A380/BmiBr | 5.7 | 17.8 | 12.1 | 0.5 | 2.3 |
| A380/BmpyrBr | 6.1 | 17.2 | 11.1 | 0.6 | 2.4 |
| NR Compounds | Tg (°C) | ∆Cp (J/g × K) | Temperature of Vulcanization (°C) | ∆H (J/g) |
|---|---|---|---|---|
| Unfilled sample | −63.8 | 0.43 | 147–215 | 6.4 |
| Hydrotalcite | ||||
| HTA | −64.6 | 0.34 | 126–213 | 14.7 |
| HTA/BmiBr | −63.9 | 0.32 | 128–235 | 18.7 |
| HTA/BmpyrBr | −64.1 | 0.35 | 126–237 | 13.9 |
| Cellulose | ||||
| CE | −63.2 | 0.33 | 129–232 | 12.0 |
| CE/BmiBr | −64.1 | 0.35 | 126–238 | 16.6 |
| CE/BmpyrBr | −64.8 | 0.36 | 128–232 | 13.0 |
| Silica A380 | ||||
| A380 | −63.9 | 0.31 | 149–232 | 12.0 |
| A380/BmiBr | −64.2 | 0.32 | 146–219 | 10.9 |
| A380/BmpyrBr | −64.4 | 0.35 | 149–216 | 12.7 |
| NR Vulcanizates | SE300 (MPa) | TS (MPa) | EB (%) | H (ShA) |
|---|---|---|---|---|
| Unfilled sample | 2.5 ± 0.4 | 17.4 ± 1.0 | 830 ± 24 | 30 ± 1 |
| Hydrotalcite | ||||
| HTA | 3.4 ± 0.3 | 20.1 ± 0.6 | 623 ± 18 | 37 ± 1 |
| HTA/BmiBr | 6.1 ± 0.1 | 23.8 ± 1.6 | 527 ± 16 | 41 ± 1 |
| HTA/BmpyrBr | 5.0 ± 0.2 | 21.7 ± 1.4 | 601 ± 14 | 42 ± 1 |
| Cellulose | ||||
| CE | 1.6 ± 0.3 | 9.1 ± 0.6 | 629 ± 18 | 42 ± 1 |
| CE/BmiBr | 2.6 ± 0.1 | 10.7 ± 0.2 | 578 ± 4 | 43 ± 1 |
| CE/BmpyrBr | 2.5 ± 0.1 | 10.2 ± 0.5 | 610 ± 16 | 44 ± 1 |
| Silica A380 | ||||
| A380 | 3.2 ± 0.1 | 8.7 ± 0.2 | 634 ± 21 | 49 ± 1 |
| A380/BmiBr | 4.4 ± 0.1 | 13.4 ± 0.9 | 599 ± 16 | 52 ± 1 |
| A380/BmpyrBr | 4.6 ± 0.3 | 11.7 ± 0.5 | 526 ± 18 | 52 ± 1 |
| NR Vulcanizates | Tg (°C) | Tan δTg (-) | Tan δ25 °C (-) | Tan δ50 °C (-) |
|---|---|---|---|---|
| Unfilled sample | −68.9 | 2.5 | 0.04 | 0.04 |
| Hydrotalcite | ||||
| HTA | −68.2 | 2.2 | 0.03 | 0.03 |
| HTA/BmiBr | −71.1 | 2.1 | 0.03 | 0.03 |
| HTA/BmpyrBr | −71.5 | 2.0 | 0.03 | 0.03 |
| Cellulose | ||||
| CE | −67.8 | 1.8 | 0.04 | 0.05 |
| CE/BmiBr | −68.9 | 1.7 | 0.05 | 0.04 |
| CE/BmpyrBr | −69.0 | 1.8 | 0.04 | 0.05 |
| Silica A380 | ||||
| A380 | −70.9 | 1.0 | 0.10 | 0.10 |
| A380/BmiBr | −69.8 | 1.0 | 0.09 | 0.10 |
| A380/BmpyrBr | −71.5 | 1.0 | 0.10 | 0.10 |
| NR Vulcanizates | T5% (°C) | TDTG (°C) | ∆m25–600 °C (%) | ∆m600–800 °C (%) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| Unfilled sample | 310 | 402 | 94.7 | 1.3 | 4.0 |
| Hydrotalcite | |||||
| HTA | 284 | 396 | 83.5 | 0.9 | 15.6 |
| HTA/BmiBr | 271 | 396 | 83.5 | 1.8 | 14.7 |
| HTA/BmpyrBr | 263 | 400 | 83.0 | 1.8 | 15.2 |
| Cellulose | |||||
| CE | 317 | 398 | 94.8 | 2.1 | 3.1 |
| CE/BmiBr | 294 | 399 | 93.7 | 3.2 | 3.1 |
| CE/BmpyrBr | 288 | 399 | 92.7 | 3.3 | 4.0 |
| Silica A380 | |||||
| A380 | 335 | 401 | 74.5 | 1.6 | 23.9 |
| A380/BmiBr | 323 | 400 | 75.6 | 2.4 | 22.0 |
| A380/BmpyrBr | 320 | 398 | 75.5 | 2.3 | 22.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska, M.; Sowińska, A. Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites. Polymers 2021, 13, 1656. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101656
Maciejewska M, Sowińska A. Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites. Polymers. 2021; 13(10):1656. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101656
Chicago/Turabian StyleMaciejewska, Magdalena, and Anna Sowińska. 2021. "Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites" Polymers 13, no. 10: 1656. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101656