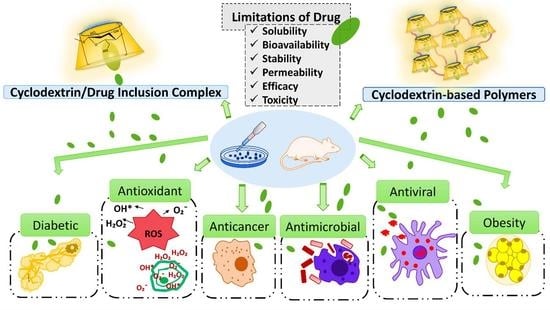
Cyclodextrin Monomers and Polymers for Drug Activity Enhancement
Abstract
:1. Introduction
2. Cyclodextrin Monomers and Polymers as Drugs Themselves
2.1. Anticholesterol Effect
2.2. Cyclodextrins as an Active Diet Agent
3. Cyclodextrin Monomers and Polymers as an Enhancer of the Drug Effect
3.1. Antimicrobial Activity
3.2. Antiviral Activity
3.3. Cardiovascular Activity
3.4. Neurological Diseases
3.4.1. Alzheimer’s Disease
3.4.2. Parkinson’s Disease
3.5. Anticancer Activity
3.6. Antioxidant Activity
3.7. Diabetes Activity
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-Nicolás, J.M.; Trotta, F. Cyclic oligosaccharides as active drugs, an updated review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; González-Ramón, A.; García-Carmona, F.; López-Nicolás, J.M. Recent advances in the treatment of Niemann pick disease type C: A mini-review. Int. J. Pharm. 2020, 584, 119440. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Aggregation of t10,c12 conjugated linoleic Acid in presence of natural and modified cyclodextrins. A physicochemical, thermal and computational analysis. Chem. Phys. Lipids 2017, 204, 57–64. [Google Scholar] [CrossRef]
- Matencio, A.; Bermejo-Gimeno, M.J.; García-Carmona, F.; López-Nicolás, J.M. Separating and identifying the four stereoisomers of methyl jasmonate by RP-HPLC and using cyclodextrins in a novel way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Guerrero-Rubio, M.A.; Gandía-Herrero, F.; García-Carmona, F.; López-Nicolás, J.M. Nanoparticles of betalamic acid derivatives with cyclodextrins. Physicochemistry, production characterization and stability. Food Hydrocoll. 2021, 110, 106176. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Matencio, A.; Vicente-Herrero, C.; García-Carmona, F.; López-Nicolás, J.M. Study of the fluorescence and interaction between cyclodextrins and neochlorogenic acid, in comparison with chlorogenic acid. Sci. Rep. 2021, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Caldera, F.; Pedrazzo, A.R.; Monfared, Y.K.; Kumar-Dhakar, N.; Trotta, F. A physicochemical, thermodynamical, structural and computational evaluation of kynurenic acid/cyclodextrin complexes. Food Chem. 2021, 356, 129639. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Effect of hydroxypropyl-β-cyclodextrin on the aggregation of (E)-resveratrol in different protonation states of the guest molecule. Food Chem. 2010, 118, 648–655. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Liu, Y.; Zhao, L.; Fu, Y.; Ye, F. Enhanced physicochemical properties and herbicidal activity of an environment-friendly clathrate formed by β-cyclodextrin and herbicide cyanazine. J. Mol. Liq. 2020, 305, 112858. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. An environmentally safe formulation with enhanced solubility and fungicidal activity: Self-assembly and characterization of Difenoconazole-β-CD inclusion complex. J. Mol. Liq. 2021, 327, 114874. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of aromatic chlorinated pesticides from aqueous solution using β-cyclodextrin polymers decorated with Fe3O4 Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.B.; Duarte, F.; Ílary, C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; Neves de Lima, Á.A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; Conesa, I.; Muñoz-Sánchez, I.; Laveda-Cano, L.; Cano-Yelo, D.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of juice and milk “food models” fortified with oxyresveratrol and β-Cyclodextrin. Food Hydrocoll. 2020, 98, 105250. [Google Scholar] [CrossRef]
- Matencio, A.; Hernández-García, S.; García-Carmona, F.; López-Nicolás, J.M. A way to increase the bioaccesibility and photostability of roflumilast, a COPD treatment, by cyclodextrin monomers. Polymers 2019, 11, 801. [Google Scholar] [CrossRef] [Green Version]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative evaluation of solubility, cytotoxicity and photostability studies of resveratrol and oxyresveratrol loaded nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [Green Version]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Liu, J. The classification and application of cyclodextrin polymers: A review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Williams, R.O.; Mahaguna, V.; Sriwongjanya, M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-beta-cyclodextrin. Eur. J. Pharm. Biopharm. 1998, 46, 355–360. [Google Scholar] [CrossRef]
- Evans, W.R.H.; Hendriksz, C.J. Niemann-Pick type C disease—The tip of the iceberg? A review of neuropsychiatric presentation, diagnosis and treatment. BJPsych Bull. 2017, 41, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Hao, J.; Fujiwara, H.; Xu, M.; Yang, S.; Dai, S.; Long, Y.; Swaroop, M.; Li, C.; Vu, M.; et al. Analytical characterization of methyl-β-cyclodextrin for pharmacological activity to reduce lysosomal cholesterol accumulation in niemann-pick disease type C1 Cells. Assay Drug Dev. Technol. 2017, 15, 154–166. [Google Scholar] [CrossRef]
- Matencio, A.; Alcaráz-Gómez, M.A.; García-Carmona, F.; Arias, B.; López-Nicolás, J.M. Application of a simple methodology to analyze Hydroxypropyl-β-Cyclodextrin in urine using HPLC–LS in early Niemann–Pick disease type C patient. J. Chromatogr. B 2018, 1093–1094, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.-L.; Diana, V.G.; Fabian, S.-G.; Carmen, F.-L.; Helios, P.-G.; Nuria, G.; Gisela, N.G.; Alejandro, L. Niemann-Pick disease treatment: A systematic review of clinical trials. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Singhal, A.; Szente, L.; Hildreth, J.E.K.; Song, B. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann–Pick C1 mutant cell via lysosome-associated membrane protein 1. Cell Death Dis. 2018, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Pilely, K.; Bakke, S.S.; Palarasah, Y.; Skjoedt, M.-O.; Bartels, E.D.; Espevik, T.; Garred, P. Alpha-cyclodextrin inhibits cholesterol crystal-induced complement-mediated inflammation: A potential new compound for treatment of atherosclerosis. Atherosclerosis 2019, 283, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the impact of cavity size, occupancy, and substitutions on cytotoxicity and cholesterol homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmin, N.; Ishitsuka, Y.; Fukaura, M.; Yamada, Y.; Nakahara, S.; Ishii, A.; Kondo, Y.; Takeo, T.; Nakagata, N.; Motoyama, K.; et al. In vitro and in vivo evaluation of 6-O-α-Maltosyl-β-Cyclodextrin as a potential therapeutic agent against niemann-pick Disease Type C. Int. J. Mol. Sci. 2019, 20, 1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoyama, K.; Hirai, Y.; Nishiyama, R.; Maeda, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Era, T.; Arima, H. Cholesterol lowering effects of mono-lactose-appended β-cyclodextrin in Niemann–Pick type C disease-like HepG2 cells. Beilstein J. Org. Chem. 2015, 11, 2079–2086. [Google Scholar] [CrossRef]
- Motoyama, K.; Nishiyama, R.; Maeda, Y.; Higashi, T.; Kawaguchi, Y.; Futaki, S.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Era, T.; et al. Cholesterol-lowering effect of octaarginine-appended β-Cyclodextrin in Npc1-Trap-CHO Cells. Biol. Pharm. Bull. 2016, 39, 1823–1829. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, A.; Yagci, Y. Cyclodextrin-based macromolecular systems as cholesterol-mopping therapeutic agents in niemann-pick Disease Type C. Macromol. Rapid Commun. 2018, 40, e1800557. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Caporali, P.; Dolas, A.; Johny, S.; Goyal, S.; Dragotto, J.; Macone, A.; Jayaraman, R.; Fiorenza, M.T. Linear Cyclodextrin polymer prodrugs as novel therapeutics for niemann-pick Type C1 Disorder. Sci. Rep. 2018, 8, 9547. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Whittaker, G.R. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.K.; Gupta, D.; Lal, S.K. Host lipid rafts play a major role in binding and endocytosis of influenza a virus. Viruses 2018, 10, 650. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Xiao, S.; Zhou, D.; Sollogoub, M.; Zhang, Y. Design, synthesis and biological evaluation of water-soluble per-O-methylated cyclodextrin-C60 conjugates as anti-influenza virus agents. Eur. J. Med. Chem. 2018, 146, 194–205. [Google Scholar] [CrossRef]
- Tian, Z.; Si, L.; Meng, K.; Zhou, X.; Zhang, Y.; Zhou, D.; Xiao, S. Inhibition of influenza virus infection by multivalent pentacyclic triterpene-functionalized per- O -methylated cyclodextrin conjugates. Eur. J. Med. Chem. 2017, 134, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Si, L.; Tian, Z.; Jiao, P.; Fan, Z.; Meng, K.; Zhou, X.; Wang, H.; Xu, R.; Han, X.; et al. Pentacyclic triterpenes grafted on CD cores to interfere with influenza virus entry: A dramatic multivalent effect. Biomaterials 2016, 78, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Schneider, S.M.; Nicola, A.V. Herpes Simplex Virus 1 Envelope Cholesterol Facilitates Membrane Fusion. Front. Microbiol. 2017, 8, 2383. [Google Scholar] [CrossRef] [Green Version]
- Hambleton, S.; Steinberg, S.P.; Gershon, M.D.; Gershon, A.A. Cholesterol Dependence of Varicella-Zoster Virion Entry into Target Cells. J. Virol. 2007, 81, 7548–7558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, S.; Saravanabalaji, D.; Yi, M. Detergent-Resistant Membrane Association of NS2 and E2 during Hepatitis C Virus Replication. J. Virol. 2015, 89, 4562–4574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mingxue, B.; Chaolumen, B.; Asai, D.; Miyazaki, K.; Yoshida, T. Synthesis and Anti-HIV Activity of Sulfated Oligosaccharide-Branched β-CD. J. Fiber Sci. Technol. 2020, 76, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Mingxue, B.; Chaolumen, B.; Asai, D.; Takemura, H.; Miyazaki, K.; Yoshida, T. Role of a long-chain alkyl group in sulfated alkyl oligosaccharides with high anti-HIV activity revealed by SPR and DLS. Carbohydr. Polym. 2020, 245, 116518. [Google Scholar] [CrossRef]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L.; et al. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef] [Green Version]
- Férézou, J.; Riottot, M.; Sérougne, C.; Cohen-Solal, C.; Catala, I.B.; Alquier, C.; Parquet, M.; Juste, C.; Lafont, H.; Mathé, D.; et al. Hypocholesterolemic action of beta-cyclodextrin and its effects on cholesterol metabolism in pigs fed a cholesterol-enriched diet. J. Lipid Res. 1997, 38, 86–100. [Google Scholar] [CrossRef]
- Wagner, E.M.; Jen, K.-L.C.; Artiss, J.D.; Remaley, A.T. Dietary α-cyclodextrin lowers low-density lipoprotein cholesterol and alters plasma fatty acid profile in low-density lipoprotein receptor knockout mice on a high-fat diet. Metabolism 2008, 57, 1046–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Burk, J.; Picone, T.; Hendy, C.; Tan, M.M.C.; Caterson, I.D. Effects of alpha-cyclodextrin on cholesterol control and Compound K on glycaemic control in people with pre-diabetes: Protocol for a Phase III randomized controlled trial. Clin. Obes. 2019, 9, e12324. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.A.; Fletcher, E.; Elserafy, E.; Artiss, J.D.; Jen, K.-L.C. The Effect of α-Cyclodextrin on postprandial lipid and glycemic responses to a fat-containing meal. Metabolism 2013, 62, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. BioFactors 2018, 44, 336–347. [Google Scholar] [CrossRef]
- Wupper, S.; Fisher, A.; Luersen, K.; Ipharraguerre, I.R.; Chikamoto, K.; Furune, T.; Ishida, Y.; Terao, K.; Rimbach, G. Effects of dietary gamma-cyclodextrin on voluntary activity and muscle strength in Mice. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to alpha cyclodextrin and reduction of post prandial glycaemic responses (ID 2926, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2713. [Google Scholar] [CrossRef] [Green Version]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [Green Version]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides films of low-density polyethylene (LDPE)/inclusion complexes (Carvacrol and Cinnamaldehyde) against botrytis cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, C.A.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Ruellan, S.; Fourmentin, S. Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food Chem. 2014, 164, 454–461. [Google Scholar] [CrossRef]
- Andrade, P.F.; de Faria, A.F.; da Silva, D.S.; Bonacin, J.A.; Gonçalves, M.D.C. Structural and morphological investigations of β-cyclodextrin-coated silver nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 289–297. [Google Scholar] [CrossRef]
- Uyar, T.; Hacaloglu, J.; Besenbacher, F. Electrospun polystyrene fibers containing high temperature stable volatile fragrance/flavor facilitated by cyclodextrin inclusion complexes. React. Funct. Polym. 2009, 69, 145–150. [Google Scholar] [CrossRef]
- Sun, X.; Sui, S.; Ference, C.; Zhang, Y.; Sun, S.; Zhou, N.; Zhu, W.; Zhou, K. Antimicrobial and mechanical properties of β-cyclodextrin inclusion with essential oils in chitosan films. J. Agric. Food Chem. 2014, 62, 8914–8918. [Google Scholar] [CrossRef]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Mantilla, N.; Castell-Perez, M.; Gomes, C.; Moreira, R. Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT 2013, 51, 37–43. [Google Scholar] [CrossRef]
- Brasil, I.; Gomes, C.; Puerta-Gomez, A.; Castell-Perez, M.; Moreira, R. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT 2012, 47, 39–45. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Ye, F.; Fu, Y.; Zhao, L. Thiram/hydroxypropyl-β-cyclodextrin inclusion complex electrospun nanofibers for a fast dissolving water-based drug delivery system. Colloids Surf. B Biointerfaces 2021, 201, 111625. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Zhao, L.; Fu, Y.; Ye, F. Encapsulation of thiabendazole in hydroxypropyl- β -cyclodextrin nanofibers via polymer-free electrospinning and its characterization. Pest. Manag. Sci. 2020, 76, 3264–3272. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem. 2016, 197, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.C.; Nerín, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of babchi oil in cyclodextrin-based nanosponges: Physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Deshmukh, K.; Tanwar, Y.S.; Sharma, S.; Shende, P.; Cavalli, R. Functionalized nanosponges for controlled antibacterial and antihypocalcemic actions. Biomed. Pharmacother. 2016, 84, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Shende, P. Drug-free cyclodextrin-based nanosponges for antimicrobial activity. J. Pharm. Innov. 2020, 1–11. [Google Scholar] [CrossRef]
- Sathigari, S.; Chadha, G.; Lee, Y.-H.P.; Wright, N.; Parsons, D.L.; Rangari, V.K.; Fasina, O.; Babu, R.J. Physicochemical characterization of efavirenz–cyclodextrin inclusion complexes. AAPS PharmSciTech 2009, 10, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Rossel, C.V.P.; Carreño, J.S.; Rodríguez-Baeza, M.; Alderete, J.B. Inclusion complex of the antiviral drug acyclovir with cyclodextrin in aqueous solution and in solid phase. Química Nova 2000, 23, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Goyal, G.; Vavia, P.R. Complexation approach for fixed dose tablet formulation of lopinavir and ritonavir: An anomalous relationship between stability constant, dissolution rate and saturation solubility. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 75–85. [Google Scholar] [CrossRef]
- Nicolazzi, C.; Venard, V.; Le Faou, A. Chantal Finance In vitro antiviral efficacy of the ganciclovir complexed with β-cyclodextrin on human cytomegalovirus clinical strains. Antivir. Res. 2002, 54, 121–127. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Shirsath, C. Enhancement of Bioavailability of Non-nucleoside Reverse Transciptase Inhibitor Using Nanosponges. AAPS PharmSciTech 2016, 18, 1728–1738. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech 2018, 19, 2358–2369. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Piperno, A.; Zagami, R.; Cordaro, A.; Pennisi, R.; Musarra-Pizzo, M.; Scala, A.; Sciortino, M.T.; Mazzaglia, A. Exploring the entrapment of antiviral agents in hyaluronic acid-cyclodextrin conjugates. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 33–40. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Argenziano, M.; Rittà, M.; Bastiancich, C.; Civra, A.; Lembo, D.; Cavalli, R. Acyclovir-loaded sulfobutyl ether-β-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. Int. J. Pharm. 2020, 587, 119676. [Google Scholar] [CrossRef]
- Seddon, A.M. Materials Science in the time of Coronavirus. J. Mater. Sci. 2020, 55, 9145–9147. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.B.; Simões, L.O.; Medeiros, C.F.D.A.; Jesus, A.D.M.; Fregoneze, J.B.; Evangelista, A.; Villarreal, C.F.; Araújo, A.A.D.S.; Quintans-Júnior, L.J.; Silva, D.F. Antihypertensive potential of linalool and linalool complexed with β-cyclodextrin: Effects of subchronic treatment on blood pressure and vascular reactivity. Biochem. Pharmacol. 2018, 151, 38–46. [Google Scholar] [CrossRef]
- Moreira, I.J.A.; Menezes, P.P.; Serafini, M.R.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Bonjardim, L.R.; Filho, V.J.S.; Júnior, D.B.P.; Santos, S.L.; Júnior, W.L.; et al. Characterization and antihypertensive effect of the complex of (-)-β- Pinene in β-Cyclodextrin. Curr. Pharm. Biotechnol. 2016, 17, 837–845. [Google Scholar] [CrossRef]
- Mollica, J.A.; Rehm, C.R.; Smith, J.B.; Govan, H.K. Hydrolysis of Benzothiadiazines. J. Pharm. Sci. 1971, 60, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Mennini, N.; Mannelli, L.D.C.; Micheli, L.; Ghelardini, C.; Mura, P. Development of a stable oral pediatric solution of hydrochlorothiazide by the combined use of cyclodextrins and hydrophilic polymers. Int. J. Pharm. 2020, 587, 119692. [Google Scholar] [CrossRef] [PubMed]
- Femminò, S.; Penna, C.; Bessone, F.; Caldera, F.; Dhakar, N.K.; Cau, D.; Pagliaro, P.; Cavalli, R.; Trotta, F. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers 2018, 10, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluijter, J.; Condorelli, G.; Davidson, S.M.; Engel, F.B.; Ferdinandy, P.; Hausenloy, D.J.; Lecour, S.; Madonna, R.; Ovize, M.; Ruiz-Meana, M.; et al. Novel therapeutic strategies for cardioprotection. Pharmacol. Ther. 2014, 144, 60–70. [Google Scholar] [CrossRef] [PubMed]
- El Kadmiri, N.; Said, N.; Slassi, I.; El Moutawakil, B.; Nadifi, S. Biomarkers for Alzheimer Disease: Classical and novel candidates’ review. Neuroscience 2018, 370, 181–190. [Google Scholar] [CrossRef]
- Finch, C.E.; Cohen, D.M. Aging, metabolism, and Alzheimer Disease: Review and hypotheses. Exp. Neurol. 1997, 143, 82–102. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Petrov, D.; Abad, S.; Pedrós, I.; Marin, M.; Olloquequi, J.; Camins, A. Review of the advances in treatment for Alzheimer disease: Strategies for combating β-amyloid protein. Neurología 2018, 33, 47–58. [Google Scholar] [CrossRef]
- Camilleri, P.; Haskins, N.J.; Hewlett, D.R. β-Cyclodextrin interacts with the Alzheimer amyloid β-A4 peptide. FEBS Lett. 1994, 341, 256–258. [Google Scholar] [CrossRef] [Green Version]
- Danielsson, J.; Jarvet, J.; Damberg, A.P.; Gräslund, A. Two-site binding of β-cyclodextrin to the Alzheimer aβ(1–40) peptide measured with combined pfg-nmr diffusion and induced chemical shifts. Biochememistry 2004, 43, 6261–6269. [Google Scholar] [CrossRef]
- Ren, B.; Jiang, B.; Hu, R.; Zhang, M.; Chen, H.; Ma, J.; Sun, Y.; Jia, L.; Zheng, J. HP-β-cyclodextrin as an inhibitor of amyloid-β aggregation and toxicity. Phys. Chem. Chem. Phys. 2016, 18, 20476–20485. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Pan, Y.; Yan, Y.; Hu, X.; Chen, R.; Ravoo, B.J.; Guo, D.; Zhang, T. Recognition and removal of amyloid-β by a heteromultivalent macrocyclic coassembly: A potential strategy for the treatment of Alzheimer’s Disease. Adv. Mater. 2021, 33, e2006483. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, X.; Liu, F.; Zheng, J.; Sun, Y. Ac-LVFFARK-NH 2 conjugation to β-cyclodextrin exhibits significantly enhanced performance on inhibiting amyloid β-protein fibrillogenesis and cytotoxicity. Biophys. Chem. 2018, 235, 40–47. [Google Scholar] [CrossRef]
- Wong, K.H.; Xie, Y.; Huang, X.; Kadota, K.; Yao, X.-S.; Yu, Y.; Chen, X.; Lu, A.; Yang, Z. Delivering crocetin across the blood-brain barrier by using γ-cyclodextrin to treat Alzheimer’s Disease. Sci. Rep. 2020, 10, 3654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.-L. In vitro and in vivo comparison of curcumin-encapsulated chitosan-coated poly(lactic-co-glycolic acid) nanoparticles and curcumin/hydroxypropyl-β-Cyclodextrin inclusion complexes administered intranasally as therapeutic strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the cholesterol-lowering compound methyl-β-cyclodextrin in models of α-synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef]
- Barros, M.C.F.; Ribeiro, A.C.F.; Esteso, M.A. Cyclodextrins in Parkinson’s Disease. Biomolecules 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotta, F.; Caldera, F.; Cavalli, R.; Soster, M.; Riedo, C.; Biasizzo, M.; Barretta, G.U.; Balzano, F.; Brunella, V. Molecularly imprinted cyclodextrin nanosponges for the controlled delivery of L-DOPA: Perspectives for the treatment of Parkinson’s disease. Expert Opin. Drug Deliv. 2016, 13, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kumar, V.; Yang, D.; Chowdhury, P.R.; Hohl, R.J. Cyclodextrin complexation: Influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur. J. Pharm. Sci. 2002, 15, 163–170. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, U.S.; Balasubramanian, S.V.; Straubinger, R.M. Pharmaceutical and Physical Properties of Paclitaxel (Taxol †) Complexes with Cyclodextrins. J. Pharm. Sci. 1995, 84, 1223–1230. [Google Scholar] [CrossRef]
- Erdoğar, N.; Nemutlu, E.; Iskit, A.B.; Kara, S.C.; Teksin, Z.Ş.; Bilensoy, E. Improved oral bioavailability of anticancer drug tamoxifen through complexation with water soluble cyclodextrins: In vitro and in vivo evaluation. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 81–91. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, I.; Lopez-Nicolas, J.M.; Núñez-Delicado, E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007, 104, 39–44. [Google Scholar] [CrossRef]
- Venuti, V.; Cannavà, C.; Cristiano, M.C.; Fresta, M.; Majolino, D.; Paolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B Biointerfaces 2014, 115, 22–28. [Google Scholar] [CrossRef]
- Sri, K.V.; Kondaiah, A.; Ratna, J.V.; Annapurna, A. Preparation and characterization of quercetin and rutin cyclodextrin inclusion complexes. Drug Dev. Ind. Pharm. 2007, 33, 245–253. [Google Scholar] [CrossRef]
- Gürten, B.; Yenigül, E.; Sezer, A.D.; Malta, S. Complexation and enhancement of temozolomide solubility with cyclodextrins. Braz. J. Pharm. Sci. 2018, 54, 1–11. [Google Scholar] [CrossRef]
- Neacșu, A. Physicochemical investigation of the complexation between γ-cyclodextrin and doxorubicin in solution and in solid state. Thermochim. Acta 2018, 661, 51–58. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Jiang, K.; Li, K.; Cong, Y.; Pu, S.; Jin, Y.; Lin, J. Preparation, characterisation and antitumour activity of β-, γ- and HP-β-cyclodextrin inclusion complexes of oxaliplatin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Nasongkla, N.; Wiedmann, A.F.; Bruening, A.; Beman, M.; Ray, D.; Bornmann, W.G.; Boothman, D.A.; Gao, J. Enhancement of solubility and bioavailability of β-lapachone using cyclodextrin inclusion complexes. Pharm. Res. 2003, 20, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, R.M.; Cutrignelli, A.; Stefanachi, A.; Porcelli, L.; Lopedota, A.A.; Di Fonte, R.; Lopalco, A.; Serratì, S.; Laquintana, V.; Silvestris, N.; et al. Hydroxy-propil-β-cyclodextrin inclusion complexes of two biphenylnicotinamide derivatives: Formulation and anti-proliferative activity evaluation in pancreatic cancer cell models. Int. J. Mol. Sci. 2020, 21, 6545. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.; Liu, X.; Thenmozhiyal, J.; Ho, P. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur. J. Pharm. Sci. 2005, 25, 49–56. [Google Scholar] [CrossRef]
- Xiao, C.-F.; Li, K.; Huang, R.; He, G.-J.; Zhang, J.-Q.; Zhu, L.; Yang, Q.-Y.; Jiang, K.-M.; Jin, Y.; Lin, J. Investigation of inclusion complex of Epothilone A with cyclodextrins. Carbohydr. Polym. 2014, 102, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E.; Gürkaynak, O.; Ertan, M.; Şen, M.; Hıncal, A.A. Development of nonsurfactant cyclodextrin nanoparticles loaded with anticancer drug paclitaxel. J. Pharm. Sci. 2008, 97, 1519–1529. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue CDF and β-Cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and evaluation of cyclodextrin complexes for improved anticancer activity of repurposed drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [Green Version]
- De Jesus, M.B.; Fraceto, L.; Martini, M.F.; Pickholz, M.; Ferreira, C.V.; de Paula, E. Non-inclusion complexes between riboflavin and cyclodextrins. J. Pharm. Pharmacol. 2012, 64, 832–842. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [Green Version]
- Fleury, F.; Kudelina, I.; Nabiev, I. Interactions of Lactone, Carboxylate and Self-Aggregated Forms of Camptothecin with Human and Bovine Serum Albumins. FEBS Lett. 1997, 406, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Yount, G.; Yang, Y.; Wong, B.; Wang, H.-J.; Yang, L.-X. A novel camptothecin analog with enhanced antitumor activity. Anticancer Res. 2007, 27, 3173–3178. [Google Scholar]
- Cheng, J.; Khin, K.T.; Jensen, G.S.; Liu, A.; Davis, M.E. Synthesis of Linear, β-Cyclodextrin-Based Polymers and Their Camptothecin Conjugates. Bioconjug. Chem. 2003, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Giglio, V.; Viale, M.; Bertone, V.; Maric, I.; Vaccarone, R.; Vecchio, G. Cyclodextrin polymers as nanocarriers for sorafenib. Investig. New Drugs 2018, 36, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Viale, M.; Tosto, R.; Giglio, V.; Pappalardo, G.; Oliveri, V.; Maric, I.; Mariggiò, M.A.; Vecchio, G. Cyclodextrin polymers decorated with RGD peptide as delivery systems for targeted anti-cancer chemotherapy. Investig. New Drugs 2018, 37, 771–778. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Çirpanli, Y.; Bilensoy, E.; Doğan, A.L.; Çaliş, S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Minelli, R.; Cavalli, R.; Ellis, L.; Pettazzoni, P.; Trotta, F.; Ciamporcero, E.; Barrera, G.; Fantozzi, R.; Dianzani, C.; Pili, R. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur. J. Pharm. Sci. 2012, 47, 686–694. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Ansari, K.A.; Torne, S.J.; Vavia, P.P.R.; Trotta, F.; Cavalli, R. Paclitaxel loaded nanosponges: In-vitro characterization and cytotoxicity study on MCF-7 cell line culture. Curr. Drug Deliv. 2011, 8, 194–202. [Google Scholar] [CrossRef]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for Tamoxifen delivery. Pharm. Dev. Technol. 2011, 18, 619–625. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-Based Nanosponges for Delivery of Resveratrol: In Vitro Characterisation, Stability, Cytotoxicity and Permeation Study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the anti-tumor efficacy of doxorubicin nanosponges in in vitro and in mice bearing breast tumor models. Cancers 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergal, A.; Elmas, A.; Akyüz, G. A new type and effective approach for anti-cancer drug delivery application: Nanosponge. iMedPub J. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2013, 54, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, X.; Guo, X.; Ji, M.; Wang, J.; Cheng, C.; Chen, L.; Wen, C.; Zhang, Q. Physicochemical, antioxidant, in vitro release, and heat sealing properties of fish gelatin films incorporated with β-cyclodextrin/curcumin complexes for apple juice preservation. Food Bioprocess. Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of curcumin/cyclodextrin polymer inclusion complex and investigation on its antioxidant and antiproliferative activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin and cosolvent on the solubility and antioxidant activity of caffeic acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin inclusion complex electrospun nanofibers: Enhanced water solubility, prolonged shelf life, and photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef]
- Haley, R.M.; Zuckerman, S.T.; Dakhlallah, H.; Capadona, J.R.; Von Recum, H.A.; Ereifej, E.S. Resveratrol delivery from implanted cyclodextrin polymers provides sustained antioxidant effect on implanted neural probes. Int. J. Mol. Sci. 2020, 21, 3579. [Google Scholar] [CrossRef]
- Song, M.; Wang, H.; Chen, K.; Zhang, S.; Yu, L.; Elshazly, E.H.; Ke, L.; Gong, R. Oral insulin delivery by carboxymethyl-β-cyclodextrin-grafted chitosan nanoparticles for improving diabetic treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, S774–S782. [Google Scholar] [CrossRef] [Green Version]
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef] [PubMed]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Rescifina, A.; Surdo, E.; Cardile, V.; Avola, R.; Eleonora Graziano, A.C.; Stancanelli, R.; Tommasini, S.; Pistarà, V.; Ventura, C.A. Gemcitabine Anticancer Activity Enhancement by Water Soluble Celecoxib/Sulfobutyl Ether-β-Cyclodextrin Inclusion Complex. Carbohydr. Polym. 2019, 206, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Damon, J.K.; Sarode, A.; Kanabar, D.; Garcia, J.V.; Mitragotri, S.; Muth, A.; et al. Cyclodextrin Modified Erlotinib Loaded PLGA Nanoparticles for Improved Therapeutic Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2019, 122, 338–347. [Google Scholar] [CrossRef]
- Mognetti, B.; Barberis, A.; Marino, S.; Berta, G.; De Francia, S.; Trotta, F.; Cavalli, R. In Vitro Enhancement of Anticancer Activity of Paclitaxel by a Cremophor Free Cyclodextrin-Based Nanosponge Formulation. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 201–210. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.-L.; Yang, X.; Liao, X.-L.; Yang, J.; Zhang, J.-H.; Gao, C.-Z. Scutellarin-Cyclodextrin Conjugates: Synthesis, Characterization and Anticancer Activity. Carbohydr. Polym. 2013, 92, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Y.; Yang, Z.; Niu, R.; Gao, K.; Yang, B.; Liao, X.; Zhang, J. Solid Inclusion Complexes of Oleanolic Acid with Amino-Appended β-Cyclodextrins (ACDs): Preparation, Characterization, Water Solubility and Anticancer Activity. Mater. Sci. Eng. C 2016, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A Hyaluronic Acid/Cyclodextrin Based Injectable Hydrogel for Local Doxorubicin Delivery to Solid Tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Jia, S.; Lin, K.; Fan, G.; Yuan, J.; Yu, S.; Shi, J. Specific Modification with TPGS and Drug Loading of Cyclodextrin Polyrotaxanes and the Enhanced Antitumor Activity Study in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 46427–46436. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Nariya, P.; Joshi, A.; Vohra, A.; Devkar, R.; Seshadri, S.; Thakore, S. Carbon Nanotube Embedded Cyclodextrin Polymer Derived Injectable Nanocarrier: A Multiple Faceted Platform for Stimulation of Multi-Drug Resistance Reversal. Carbohydr. Polym. 2020, 247, 116751. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Menezes, P.M.N.; de Almeida, R.L.; Silva, F.S.; de Araújo Ribeiro, L.A.; de Silva, J.A.; de Oliveira, A.P.; da Cruz Araújo, E.C.; Rolim, L.A.; Nunes, X.P. Inclusion of Vitexin in β-Cyclodextrin: Preparation, Characterization and Expectorant/antitussive activities. Heliyon 2020, 6, e05461. [Google Scholar] [CrossRef]
- Cetin Babaoglu, H.; Bayrak, A.; Ozdemir, N.; Ozgun, N. Encapsulation of Clove Essential Oil in Hydroxypropyl Beta-Cyclodextrin for Characterization, Controlled Release, and Antioxidant Activity. J. Food Process. Preserv. 2017, 41, e13202. [Google Scholar] [CrossRef]
- Tommasini, S.; Calabrò, M.L.; Stancanelli, R.; Donato, P.; Costa, C.; Catania, S.; Villari, V.; Ficarra, P.; Ficarra, P. The Inclusion Complexes of Hesperetin and Its 7-Rhamnoglucoside with(2-Hydroxypropyl)-β-Cyclodextrin. J. Pharm. Biomed. Anal. 2005, 39, 572–580. [Google Scholar] [CrossRef]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a Myricetin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex: Preparation, Characterization, and Evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant Activity of Inclusion Complexes of Tea Catechins with β-Cyclodextrins by ORAC Assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Medronho, B.; Valente, A.J.; Costa, P.; Romano, A. Inclusion Complexes of Rosmarinic Acid and Cyclodextrins: Stoichiometry, Association Constants, and Antioxidant Potential. Colloid Polym. Sci. 2014, 292, 885–894. [Google Scholar] [CrossRef]
- Chakraborty, S.; Basu, S.; Lahiri, A.; Basak, S. Inclusion of Chrysin Inb-Cyclodextrin Nanocavity and Its Effect on Antioxidantpotential of Chrysin: A Spectroscopic and Molecular Modeling Approach. J. Mol. Struct. 2010, 977, 180–188. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In Vitro Fermentation of Anthocyanins Encapsulated with Cyclodextrins: Release, Metabolism and Influence on Gut Microbiota Growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of Gallic Acid/Cyclodextrin Inclusion Complex in Electrospun Polylactic Acid Nanofibers: Release Behavior and Antioxidant Activity of Gallic Acid. Mater. Sci. Eng. C 2016, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Carlotti, M.E.; Cavalli, R.; Ugazio, E.; Berlier, G.; Gastaldi, L.; Morel, S. Photochemical and Antioxidant Properties of Gamma-Oryzanol in Beta-Cyclodextrin-Based Nanosponges. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 69–76. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Carbonyl and Carboxylate Crosslinked Cyclodextrin as a Nanocarrier for Resveratrol: In Silico, in Vitro and in Vivo Evaluation. J. Incl. Phenom. Macrocycl. Chem. 2018, 92, 261–272. [Google Scholar] [CrossRef]

| Disease | Classic CD Monomer | CD Polymeric Material | Drug | Activity | Reference |
|---|---|---|---|---|---|
| Antimicrobial | Several CDs (HPβ- or α-CD) | - | trans-Anethone | - Improved solubility - Improved stability | [59] |
| β-CD | - | Oxyresveratrol | - Increased solubility enhancing the antimicrobial capacity | [19] | |
| Several CDs (HPβ- or α-CD) | Neochlorogenic acid | - Improved solubility - Improved stability | [9] | ||
| - | PS fibers containing CDs | Menthol | - Improved stability and durability | [61] | |
| Silver particles stabilized by β-CD | Silver particles | - Stabilizer of the particles | [60] | ||
| - | β-CD polymers of polyethylene | Carvatrol | - Enhanced antimicrobial activity | [57] | |
| - | β-CD polymer with chitosan | [62] | |||
| - | β-CD polymer with cellulose | [63] | |||
| - | HPβ-CD electrospun nanofibers | Thiabendazole | - Improved solubility - Improved stability - Improved activity | [67] | |
| Thiram | [66] | ||||
| - | HPβ-, Mβ-, and HPγ-CD electrospun nanofibers | Limonene | - Improved solubility - Improved stability - Improved activity | [68] | |
| - | CD-NS | Coriander essential oil | - Improved release | [69] | |
| - | Babchi essential oil | [71] | |||
| - | Cinnamon oil | [70] | |||
| - | Norfloxacin | - Increased in vivo antibiotic capacity and permeability | [72] | ||
| - | Lysozyme | - Absorption of the enzyme | [73] | ||
| Several CDs (HPβ- or β-CD) | - | Citronella | - Improved release - In combination with Glucobay®, increased antimicrobial capacity | [58] | |
| Antiviral | CD monomers (β-CD or HPβ-CD) | - | Acyclovir | - Increased solubility | [76] |
| - | Efavirenz | - Increased solubility - Increased bioavailability | [75], | ||
| - | Lopinavir | [77] | |||
| - | Ganciclovir | [78] | |||
| - | CD-NS | Efavirenz | - Increased bioavailability in rats | [79] | |
| - | Rilpivirine | - Increased bioavailability in rats | [80] | ||
| - | Nelfinavir | - Increased solubility | [81] | ||
| - | Acyclovir | - Increased antiviral activity against HSV-1 | [82] | ||
| - | Hyaluronic acid–CD covalent conjugates | - Novel way to deliver | [83] | ||
| HPβ-CD electrospun nanofibers | - Improved release | [84] | |||
| - | Sulfobutyl ether-β-CD decorated with nanodroplet chitosan | - Increased antiviral activity against HSV-2 | [85] | ||
| Cardiovascular | β-CD | - | Linalool | - Decreased arterial pressure more than free drug (in β-pinene, free drug effect is negligible) | [87] |
| - | β-Pinene | [88] | |||
| β-CD in lipid nanoparticles | - | Hydrochlorothiazide | - Increased bioavailability | [90] | |
| - | HPβ-CD and SBEβ-CD in PVP polymers | - Increased solubility - Increased stability | [91] | ||
| Alzheimer’s disease | β-CD and HPβ-CD | - | β-Amyloid peptides | - Prevented aggregation | [97,98,99] |
| - | Co-assembled CD/calixarene | [100] | |||
| - | LK7-β-CD | [101] | |||
| γ-CD | - | Crocetin | - Increased delivery | [102] | |
| HPβ-CD | Chitosan-coated polylactic polymer | Curcumin | - Increased stability | [103] | |
| Parkinson’s disease | Mβ-CD | - | α-Synuclein | - Prevented aggregation | [105] |
| - | CD-NS | L-Dopa | - Better controlled release - Improved stability | [107] | |
| Diabetes | - | CD-NS | Insulin | - Increased stability - Improved release - Improved bioavailability | [153] |
| Anticancer | HPβ-CD | - | Niclosamide | - Improved solubility and bioavailability | [124] |
| - | 13-cis-Retinoic acid | - Improved bioavailability | [120] | ||
| SBEβ-CD | - | Resveratrol | - Improved oral and parenteral bioavailability | [113] | |
| - | Celecoxib | - Improved the cytotoxicity of gemcitabine | [155] | ||
| - | Erlotinib | - Increased apoptosis and inhibited autophagy | [156] | ||
| - | CD-NS | Paclitaxel | - Enhanced water solubility and anticancer activity | [157] | |
| CD conjugates | - | Scutellarin | - High antiproliferative activities | [158] | |
| α-CDs | - | Oleanolic acid | - Induction of apoptosis of cancer cells | [159] | |
| - | HA/EDA/β-CD | Doxorubicin | - Localized chemotherapy of solid tumors. | [160] | |
| CD/PRs | - | 10-Hydroxycamptothecin | - Effectively suppressed tumor growth | [161] | |
| - | CD-NS | Babchi oil | - Increased the solubility, photostability, and safety | [71] | |
| - | CNTs | Curcumin and doxorubicin hydrochloride | - Enhanced the therapeutic efficacy of drugs | [162] | |
| - | β-CD polymer | Sorafenib | - Increased the bioavailability and reduced the systemic toxicity | [131] | |
| β-CD | - | Vitexin | - Increased the bioavailability and dissolution | [163] | |
| Antioxidant | HP-β-CD | - | Clove essential oil | - Increased the total phenolic content and antioxidant activity | [164] |
| - | Hesperidin and hesperetin | - Increased the solubility | [165] | ||
| - | Myricetin | - Increased solubility - Improved oral bioavailability and antioxidant activity | [166] | ||
| β-CD | - | Tea catechins | - Affected the antioxidant reactivity | [167] | |
| - | Rosmarinic acid | - Enhanced the free radical scavenging ability and the storage stability | [168] | ||
| - | Chrysin | - Increased the antioxidant potential | [169] | ||
| - | Anthocyanins | - Improved bioavailability | [170] | ||
| PLA/HP-β-CD/ | - | Gallic acid | - High antioxidant activity | [171] | |
| - | CD-NS | Gamma-oryzanol | - Increased its potential as carrier | [172] | |
| - | Resveratrol | - Increased the oral bioavailability | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111684
Matencio A, Hoti G, Monfared YK, Rezayat A, Pedrazzo AR, Caldera F, Trotta F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers. 2021; 13(11):1684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111684
Chicago/Turabian StyleMatencio, Adrián, Gjylije Hoti, Yousef Khazaei Monfared, Azam Rezayat, Alberto Rubin Pedrazzo, Fabrizio Caldera, and Francesco Trotta. 2021. "Cyclodextrin Monomers and Polymers for Drug Activity Enhancement" Polymers 13, no. 11: 1684. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111684