Effect of Oxidation Time on the Properties of Cellulose Nanocrystals Prepared from Balsa and Kapok Fibers Using Ammonium Persulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
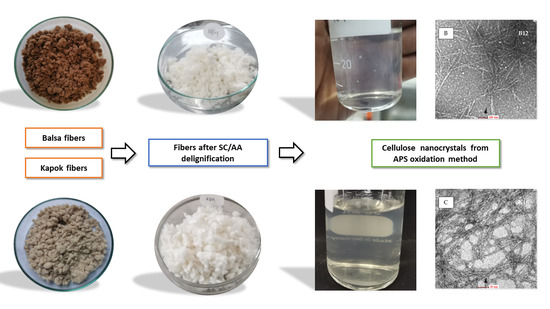
2.2. Delignification Process
2.3. Isolation of CNCs by APS Oxidation
2.4. Transmission Electron Microscopy (TEM)
2.5. Zeta Potential (ZP) Analysis
2.6. Functional Group Analysis
2.7. Crystallinity Index (CI)
2.8. Thermal-Stability Testing
3. Results and Discussion
3.1. Morphology and Particle Size Distribution of the CNCs in Suspensions
3.2. Zeta Potential
3.3. Functional Group Analysis
3.4. Crystallinity Index
3.5. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purnawati, R.; Febrianto, F.; Wistara, I.N.J.; Nikmatin, S.; Hidayat, W.; Lee, S.H.; Kim, N.H. Physical and chemical properties of kapok (Ceiba pentandra) and balsa (Ochroma pyramidale) fibers. J. Korean Wood Sci. Technol. 2018, 46, 393–401. [Google Scholar] [CrossRef]
- Marwanto, M.; Maulana, M.I.; Febrianto, F.; Wistara, N.J.; Nikmatin, S.; Masruchin, N.; Zaini, L.H.; Lee, S.-H.; Kim, N.H. Characteristics of Nanocellulose Crystals from Balsa and Kapok Fibers at Different Ammonium Persulfate Concentration. Wood Sci. Technol. 2021. under review. [Google Scholar]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef]
- Mascheroni, E.; Rampazzo, R.; Ortenzi, M.A.; Piva, G.; Bonetti, S.; Piergiovanni, L. Comparison of cellulose nanocrystals obtained by sulfuric acid hydrolysis and ammonium persulfate, to be used as coating on flexible food-packaging materials. Cellulose 2016, 23, 779–793. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-Y.; Zhang, D.-Z.; Lu, F.-F.; Yao, J. New Approach for Single-Step Extraction of Carboxylated Cellulose Nanocrystals for Their Use as Adsorbents and Flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Sun, B.; Yu, H.-Y.; Zhou, Y.; Huang, Z.; Yao, J.-M. Single-step extraction of functionalized cellulose nanocrystal and polyvinyl chloride from industrial wallpaper wastes. Ind. Crops Prod. 2016, 89, 66–77. [Google Scholar] [CrossRef]
- Niu, F.; Li, M.; Huang, Q.; Zhang, X.; Pan, W.; Yang, J.; Li, J. The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohydr. Polym. 2017, 165, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zou, Z.; Du, H.; Zhang, X.; Wang, X.; Yang, X.; Wang, H.; Li, G.; Li, L.; Si, C. Preparation of thermally stable and surface-functionalized cellulose nanocrystals via mixed H2SO4/Oxalic acid hydrolysis. Carbohydr. Polym. 2019, 223, 115116. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly Efficient and Sustainable Preparation of Carboxylic and Thermostable Cellulose Nanocrystals via FeCl3-Catalyzed Innocuous Citric Acid Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Bondancia, T.; De Aguiar, J.; Batista, G.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of Nanocellulose Using Citric Acid in a Biorefinery Concept: Effect of the Hydrolysis Reaction Time and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-Step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Oun, A.A.; Rhim, J.-W. Characterization of carboxymethyl cellulose-based nanocomposite films reinforced with oxidized nanocellulose isolated using ammonium persulfate method. Carbohydr. Polym. 2017, 174, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Culsum, N.T.U.; Melinda, C.; Leman, I.; Wibowo, A.; Budhi, Y.W. Isolation and characterization of cellulose nanocrystals (CNCs) from industrial denim waste using ammonium persulfate. Mater. Today Commun. 2021, 26, 101817. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Cao, S.-L.; Xu, P.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Preparation of a novel nanobiocatalyst by immobilizing penicillin acylase onto magnetic nanocrystalline cellulose and its use for efficient synthesis of cefaclor. Chem. Eng. J. 2018, 346, 361–368. [Google Scholar] [CrossRef]
- Fardioui, M.; Kadmiri, I.M.; Qaiss, A.E.K.; Bouhfid, R. Bio-active nanocomposite films based on nanocrystalline cellulose reinforced styrylquinoxalin-grafted-chitosan: Antibacterial and mechanical properties. Int. J. Biol. Macromol. 2018, 114, 733–740. [Google Scholar] [CrossRef]
- Karimian, A.; Parsian, H.; Majidinia, M.; Rahimi, M.; Mir, S.M.; Kafil, H.S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Ostadi, H.; Yousefi, B. Nanocrystalline cellulose: Preparation, physicochemical properties, and applications in drug delivery systems. Int. J. Biol. Macromol. 2019, 133, 850–859. [Google Scholar] [CrossRef]
- Adel, A.; El-Shafei, A.; Ibrahim, A.; Al-Shemy, M. Extraction of oxidized nanocellulose from date palm (Phoenix dactylifera L.) sheath fibers: Influence of CI and CII polymorphs on the properties of chitosan/bionanocomposite films. Ind. Crops Prod. 2018, 124, 155–165. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Bashar, M.M.; Zhu, H.; Yamamoto, S.; Mitsuishi, M. Highly carboxylated and crystalline cellulose nanocrystals from jute fiber by facile ammonium persulfate oxidation. Cellulose 2019, 26, 3671–3684. [Google Scholar] [CrossRef]
- Jang, J.-H.; Hayashi, N.; Han, S.-Y.; Park, C.-W.; Febrianto, F.; Lee, S.-H.; Kim, N.-H. Changes in the Dimensions of Lignocellulose Nanofibrils with Different Lignin Contents by Enzymatic Hydrolysis. Polymers 2020, 12, 2201. [Google Scholar] [CrossRef]
- Jin, K.; Liu, X.; Jiang, Z.; Tian, G.; Yang, S.; Shang, L.; Ma, J. Delignification kinetics and selectivity in poplar cell wall with acidified sodium chlorite. Ind. Crops Prod. 2019, 136, 87–92. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Mendoza, L.; Gunawardhana, T.; Batchelor, W.; Garnier, G. Effects of fibre dimension and charge density on nanocellulose gels. J. Colloid Interface Sci. 2018, 525, 119–125. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Hossain, L.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. Controlling the transparency and rheology of nanocellulose gels with the extent of carboxylation. Carbohydr. Polym. 2020, 245, 116566. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crops Prod. 2020, 154, 112627. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Y.; Wang, Y.; Jiang, G.; Liang, M.; Chen, X.; Long, G. A fractal model for kozeny–carman constant and dimensionless permeability of fibrous porous media with roughened surfaces. Fractals 2019, 27, 1–12. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Q.; Chen, H.; Chen, X.; Long, G. A fractal model for capillary flow through a single tortuous capillary with roughened surfaces in fibrous porous media. Fractals 2021, 29, 2150017. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Park, B.-D. Optimum oxidation for direct and efficient extraction of carboxylated cellulose nanocrystals from recycled MDF fibers by ammonium persulfate. Carbohydr. Polym. 2021, 251, 117029. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, J.H.; Easson, M.W.; Dien, B.; Thompson, S.; Condon, B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose 2019, 26, 5959–5979. [Google Scholar] [CrossRef]
- KKhanjanzadeh, B.D. Park, Characterization of carboxylated cellulose nanocrystals from recycled fiberboard fibers using ammonium persulfate oxidation. J. Korean Wood Sci. Technol. 2020, 48, 231–244. [Google Scholar] [CrossRef]
- Goh, K.Y.; Ching, Y.C.; Chuah, C.H.; Abdullah, L.C.; Liou, N.-S. Individualization of microfibrillated celluloses from oil palm empty fruit bunch: Comparative studies between acid hydrolysis and ammonium persulfate oxidation. Cellulose 2016, 23, 379–390. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: Rice straw and poplar wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Filipova, I.; Fridrihsone, V.; Cabulis, U.; Berzins, A. Synthesis of Nanofibrillated Cellulose by Combined Ammonium Persulphate Treatment with Ultrasound and Mechanical Processing. Nanomaterials 2018, 8, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaini, L.H.; Febrianto, F.; Wistara, I.N.J.; Marwanto, N.; Maulana, M.I.; Lee, S.H.; Kim, N.H. Effect of ammonium persulfate concentration on characteristics of cellulose nanocrystals from oil palm frond. J. Korean Wood Sci. Technol. 2019, 47, 597–606. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef] [PubMed]







| Samples | Reaction Time (h) | Diameter (D, nm) | Length (L, nm) | Aspect Ratio (L/D) | ||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Ave. * | Min. | Max. | Ave. * | |||
| CNCs from Balsa | 8 | 7.35 | 19.48 | 12.64 (a0) | 115.33 | 482.48 | 274.40 (a2) | 21.72 |
| 12 | 6.25 | 16.11 | 10.44 (b0) | 182.57 | 397.60 | 261.58 (a2) | 25.06 | |
| 16 | 3.93 | 11.25 | 6.52 (c0) | 52.60 | 182.65 | 95.88 (b2) | 14.72 | |
| CNCs from Kapok | 8 | 4.62 | 13.86 | 8.77 (a1) | 189.96 | 369.81 | 247.61 (a3) | 28.23 |
| 12 | 2.78 | 9.16 | 5.54 (b1) | 93.27 | 186.91 | 136.36 (b3) | 24.61 | |
| 16 | 3.14 | 8.26 | 5.82 (b1) | 82.87 | 152.36 | 112.06 (c3) | 19.24 | |
| Sample | Reaction Time (h)/Methods | ZP/mV | Literature |
|---|---|---|---|
| CNCs from balsa | 8 | −29.93 | In this study |
| 12 | −44.23 | In this study | |
| 16 | −51.43 | In this study | |
| CNCs from kapok | 8 | −10.23 | In this study |
| 12 | −61.87 | In this study | |
| 16 | −62.27 | In this study | |
| CNCs from cotton | APS oxidation methods | −50.60 | [14] |
| CNCs from MCC | APS oxidation methods | −46.90 | [14] |
| CNCs from jute fiber | APS oxidation methods | −40.00 | [24] |
| CNCs from denim waste | APS oxidation methods | −3.53 | [17] |
| CNCs from lemon pulp | APS oxidation methods | −31.27 | [16] |
| CNCs from lemon pulp | Sulfuric acid (Acid hydrolysis) | −40.27 | [16] |
| CNCs from lemon pulp | TEMPO Oxidation | −55.67 | [16] |
| CNCs from cotton pulp | Phosphoric acid (Acid hydrolysis) | −17.03 | [36] |
| Sample | CI, ° | ||
|---|---|---|---|
| Balsa | Kapok | ||
| Original fiber | 31.40 | 35.65 | |
| CNCs | 8 h | 47.49 | 55.55 |
| 12 h | 51.00 | 52.50 | |
| 16 h | 57.65 | 60.71 | |
| Sample | Reaction Time (h) | Temperature (°C) | |
|---|---|---|---|
| Tonset | Tmax | ||
| Balsa | Original fiber | 263.80 | 342.64 |
| 8 | 219.71 | 277.87 | |
| 12 | 240.73 | 289.91 | |
| 16 | 241.33 | 291.57 | |
| Kapok | Original fiber | 254.49 | 344.80 |
| 8 | 215.35 | 261.39 | |
| 12 | 216.97 | 273.93 | |
| 16 | 233.45 | 280.64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marwanto, M.; Maulana, M.I.; Febrianto, F.; Wistara, N.J.; Nikmatin, S.; Masruchin, N.; Zaini, L.H.; Lee, S.-H.; Kim, N.-H. Effect of Oxidation Time on the Properties of Cellulose Nanocrystals Prepared from Balsa and Kapok Fibers Using Ammonium Persulfate. Polymers 2021, 13, 1894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111894
Marwanto M, Maulana MI, Febrianto F, Wistara NJ, Nikmatin S, Masruchin N, Zaini LH, Lee S-H, Kim N-H. Effect of Oxidation Time on the Properties of Cellulose Nanocrystals Prepared from Balsa and Kapok Fibers Using Ammonium Persulfate. Polymers. 2021; 13(11):1894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111894
Chicago/Turabian StyleMarwanto, Marwanto, Muhammad Iqbal Maulana, Fauzi Febrianto, Nyoman Jaya Wistara, Siti Nikmatin, Nanang Masruchin, Lukmanul Hakim Zaini, Seung-Hwan Lee, and Nam-Hun Kim. 2021. "Effect of Oxidation Time on the Properties of Cellulose Nanocrystals Prepared from Balsa and Kapok Fibers Using Ammonium Persulfate" Polymers 13, no. 11: 1894. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111894