In-Situ One-Step Direct Loading of Agents in Poly(acrylic acid) Coating Deposited by Aerosol-Assisted Open-Air Plasma
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Poly(acrylic acid) Coating Deposition
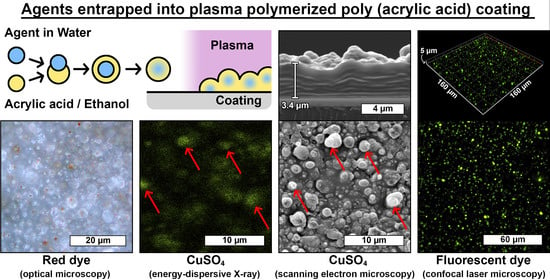
3.2. Agents Deposition and Entrapment in the Coating
3.3. Deposited Agent Quantification
3.4. Loading and Deposition Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zelikin, A.N. Drug releasing polymer thin films: New era of surface-mediated drug delivery. ACS Nano 2010, 4, 2494–2509. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.; Tan, A. Coating techniques and release kinetics of drug-eluting stents. J. Med. Devices Trans. ASME 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435-17. [Google Scholar] [CrossRef]
- John, A.A.; Subramanian, A.P.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Mohandas, H.; Paramalinggam, T.; Supriyanto, E.; Yusof, M. Review: Physico-chemical modification as a versatile strategy for the biocompatibility enhancement of biomaterials. RSC Adv. 2015, 5, 39232–39244. [Google Scholar] [CrossRef]
- Wiemer, M.; Butz, T.; Schmidt, W.; Schmitz, K.P.; Horstkotte, D.; Langer, C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: From nearly undamaged to major damaged polymers. Catheter. Cardiovasc. Interv. 2010, 75, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coatings Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Merche, D.; Vandencasteele, N.; Reniers, F. Atmospheric plasmas for thin film deposition: A critical review. Thin Solid Films 2012, 520, 4219–4236. [Google Scholar] [CrossRef]
- Mertz, G.; Fouquet, T.; El-Ahrach, H.I.; Becker, C.; Phan, T.N.T.; Ziarelli, F.; Gigmes, D.; Ruch, D. Water Sensitive Coatings Deposited by Aerosol Assisted Atmospheric Plasma Process: Tailoring the Hydrolysis Rate by the Precursor Chemistry. Plasma Process. Polym. 2015, 12, 1293–1301. [Google Scholar] [CrossRef]
- Mertz, G.; Fouquet, T.; Becker, C.; Ziarelli, F.; Ruch, D. A methacrylic anhydride difunctional precursor to produce a hydrolysis-sensitive coating by aerosol-assisted atmospheric plasma process. Plasma Process. Polym. 2014, 11, 728–733. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma polymerization-Reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; O’Neill, L.; Goodwin, A.J. Anti-microbial coatings by agent entrapment in coatings deposited via atmospheric pressure plasma liquid deposition. Surf. Interface Anal. 2006, 38, 1519–1524. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Paulussen, S.; Favia, P. Atmospheric Pressure Plasma Deposition of Poly Lactic Acid-Like Coatings with Embedded Elastin. Plasma Process. Polym. 2014, 11, 345–352. [Google Scholar] [CrossRef]
- Palumbo, F.; Porto, C.L.; Fracassi, F.; Favia, P. Recent advancements in the use of aerosol-assisted atmospheric pressure plasma deposition. Coatings 2020, 10, 440. [Google Scholar] [CrossRef]
- Heyse, P.; Roeffaers, M.B.J.; Paulussen, S.; Hofkens, J.; Jacobs, P.A.; Sels, B.F. Protein Immobilization Using Discharges: A Route to a Straightforward Manufacture of Bioactive Films. Plasma Process. Polym. 2008, 5, 186–191. [Google Scholar] [CrossRef]
- Heyse, P.; Van Hoeck, A.; Roeffaers, M.B.J.; Raffin, J.; Sto, T.; Lammertyn, J.; Verboven, P.; Jacobs, P.A.; Hofkens, J.; Paulussen, S.; et al. Exploration of Atmospheric Pressure Plasma Nanofilm Technology for Straightforward Bio-Active Coating Deposition: Enzymes, Plasmas and Polymers, an Elegant Synergy. Plasma Process. Polym. 2011, 8, 965–974. [Google Scholar] [CrossRef]
- Palumbo, F.; Camporeale, G.; Yang, Y.; Wu, J.; Sardella, E.; Dilecce, G.; Calvano, C.D.; Quintieri, L.; Caputo, L.; Baruzzi, F.; et al. Direct Plasma Deposition of Lysozyme-Embedded Bio-Composite Thin Films. Plasma Process. Polym. 2015, 12, 1302–1310. [Google Scholar] [CrossRef]
- Hsiao, C.; Wu, C.; Liu, Y.; Yang, Y.; Cheng, Y.; Palumbo, F.; Camporeale, G.; Favia, P.; Wu, J. Aerosol-Assisted Plasma Deposition of Biocomposite Coatings: Investigation of Processing Conditions on Coating Properties. IEEE Trans. Plasma Sci. 2016, 44, 3091–3098. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yang, C.H.; Lin, T.R.; Cheng, Y.C. Using aerosol-assisted atmospheric-pressure plasma to embed proteins onto a substrate in one step for biosensor fabrication. Plasma Process. Polym. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Palumbo, F.; Treglia, A.; Lo Porto, C.; Fracassi, F.; Baruzzi, F.; Frache, G.; El Assad, D.; Pistillo, B.R.; Favia, P. Plasma-Deposited Nanocapsules Containing Coatings for Drug Delivery Applications. ACS Appl. Mater. Interfaces 2018, 10, 35516–35525. [Google Scholar] [CrossRef] [PubMed]
- Lo Porto, C.; Palumbo, F.; Palazzo, G.; Favia, P. Direct plasma synthesis of nano-capsules loaded with antibiotics. Polym. Chem. 2017, 8, 1746–1749. [Google Scholar] [CrossRef]
- Lo Porto, C.; Palumbo, F.; Buxadera-Palomero, J.; Canal, C.; Jelinek, P.; Zajickova, L.; Favia, P. On the plasma deposition of vancomycin-containing nano-capsules for drug-delivery applications. Plasma Process. Polym. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Lo Porto, C.; Palumbo, F.; Fracassi, F.; Barucca, G.; Favia, P. On the formation of nanocapsules in aerosol-assisted atmospheric-pressure plasma. Plasma Process. Polym. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Lo Porto, C.; Palumbo, F.; Modic, M.; Cvelbar, U.; Ghobeira, R.; De Geyter, N.; De Vrieze, M.; Kos, Š.; Serša, G.; et al. Synthesis of antibacterial composite coating containing nanocapsules in an atmospheric pressure plasma. Mater. Sci. Eng. C 2021, 119. [Google Scholar] [CrossRef] [PubMed]
- Bitar, R.; Cools, P.; De Geyter, N.; Morent, R. Acrylic acid plasma polymerization for biomedical use. Appl. Surf. Sci. 2018, 448, 168–185. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; Van Hoeck, A.; d’Agostino, R.; Paulussen, S.; Favia, P. Atmospheric pressure plasma deposition of organic films of biomedical interest. Surf. Coat. Technol. 2011, 205, S525–S528. [Google Scholar] [CrossRef]
- Da Ponte, G.; Sardella, E.; Fanelli, F.; D’Agostino, R.; Gristina, R.; Favia, P. Plasma deposition of PEO-like coatings with aerosol-assisted dielectric barrier discharges. Plasma Process. Polym. 2012, 9, 1176–1183. [Google Scholar] [CrossRef]
- Yeo, Y.; Basaran, O.A.; Park, K. A new process for making reservoir-type microcapsules using ink-jet technology and interfacial phase separation. J. Control. Release 2003, 93, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Park, K. A new microencapsulation method using an ultrasonic atomizer based on interfacial solvent exchange. J. Control. Release 2004, 100, 379–388. [Google Scholar] [CrossRef]
- Pabari, R.M.; Sunderland, T.; Ramtoola, Z. Investigation of a Novel 3-Fluid Nozzle Spray Drying Technology for the Engineering of Multifunctional Layered Microparticles. Expert Opin. Drug Deliv. 2012, 9, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Kondo, K.; Niwa, T.; Danjo, K. Preparation of sustained-release coated particles by novel microencapsulation method using three-fluid nozzle spray drying technique. Eur. J. Pharm. Sci. 2014, 51, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Maltesen, M.J.; Andersen, S.K.; Bjerregaard, S.; Foged, C.; Rantanen, J.; Yang, M. One-Step Production of Protein-Loaded PLGA Microparticles via Spray Drying Using 3-Fluid Nozzle. Pharm. Res. 2014, 31, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Petisco-Ferrero, S.; Sánchez-Ilárduya, M.B.; Díez, A.; Martín, L.; Meaurio Arrate, E.; Sarasua, J.R. Surface functionalization of an osteoconductive filler by plasma polymerization of poly(ϵ-caprolactone) and poly(acrylic acid) films. Appl. Surf. Sci. 2016, 386, 327–336. [Google Scholar] [CrossRef]




| Condition | Water Flow Rate (µL/min) | Deposition Time (min) | LY Concentration (µg/mL) 1 |
|---|---|---|---|
| A | 0 | 5 | / |
| B | 25 | 5 | 500 |
| C | 50 | 5 | 500 |
| D | 100 | 5 | 500 |
| E | 50 | 2.5 | 500 |
| F | 50 | 10 | 500 |
| G | 50 | 5 | 250 |
| H | 50 | 5 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morand, G.; Chevallier, P.; Guyon, C.; Tatoulian, M.; Mantovani, D. In-Situ One-Step Direct Loading of Agents in Poly(acrylic acid) Coating Deposited by Aerosol-Assisted Open-Air Plasma. Polymers 2021, 13, 1931. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121931
Morand G, Chevallier P, Guyon C, Tatoulian M, Mantovani D. In-Situ One-Step Direct Loading of Agents in Poly(acrylic acid) Coating Deposited by Aerosol-Assisted Open-Air Plasma. Polymers. 2021; 13(12):1931. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121931
Chicago/Turabian StyleMorand, Gabriel, Pascale Chevallier, Cédric Guyon, Michael Tatoulian, and Diego Mantovani. 2021. "In-Situ One-Step Direct Loading of Agents in Poly(acrylic acid) Coating Deposited by Aerosol-Assisted Open-Air Plasma" Polymers 13, no. 12: 1931. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121931