Albendazole Release from Silica-Chitosan Nanospheres. In Vitro Study on Cervix Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Mesopore SiO2 Nanospheres
2.3. Albendazole Loading and Chitosan Coating of Nanospheres
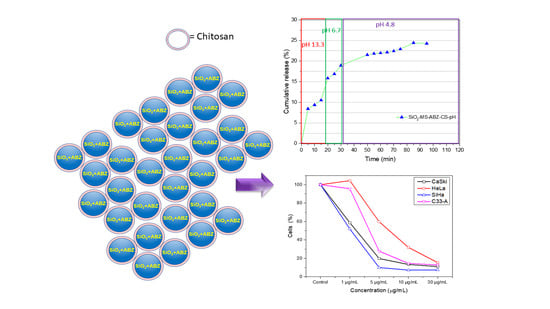
2.4. Drug Release Profiles
2.5. Characterization of the SiO2-Based Particles
2.6. Antiproliferative Performance of SiO2-MS-ABZ-CS in Cervix Cell Lines
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Drug Release Test
3.3. In Vitro Assessment of Antiproliferative Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varma, R.; Vasudevan, S. Extraction characterization, and antimicrobial activity of chitosan from Horse Mussel Modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Lee, D.; Shin, Y.; Park, J. Recent progress in polysaccharide aerogels: Their synthesis, application, and future Outlook. Polymers 2021, 13, 1347. [Google Scholar] [CrossRef] [PubMed]
- Bil, M.; Mrówka, P.; Kołbuk, D.; Święszkowski, W. Multifunctional composite combining chitosan microspheres for drug delivery embedded in shape memory polyester-urethane matrix. Compos. Sci. Technol. 2021, 201, 108481. [Google Scholar] [CrossRef]
- Su, Z.; Sun, D.; Zhang, L.; He, M.; Jiang, Y.; Millar, B.; Douglas, P.; Mariotti, D.; Maguire, P.; Sun, D. Chitosan/silver nanoparticles/graphene oxide nanocomposites with multi-drug release, antimicrobial, and photothermal conversion functions. Materials 2021, 14, 2351. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Khan Chowdhury, A.J.; Islam Sarker, M.Z. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Rostami, E. Progresses in targeted drug delivery systems using chitosan nanoparticles in cancer therapy: A mini-review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Yu, C. Mesoporous silica nanoparticles for protein protection and delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.; Hafez Ghoran, S.; Hadi Niakan, M.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah Amani, A.M. Mesoporous silica nanoparticle: Heralding a brighter future in cancer nanomedicine. Micropor. Mesopor. Mat. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef]
- Narayan, R.; Gadag, S.; Mudakavi, R.J.; Garg, S.; Raichur, A.M.; Nayak, Y.; Kini, S.G.; Ranganath Paid, K.S.; Nayak, U.Y. Mesoporous silica nanoparticles capped with chitosan-glucuronic acid conjugate for pH-responsive targeted delivery of 5-fluorouracil. J. Drug Deliv. Sci. Technol. 2021, 63, 102472. [Google Scholar] [CrossRef]
- Almomen, A.; El-Toni, A.M.; Badran, M.; Alhowyan, A.; Kalam, M.A.; Alshamsan, A.; Alkholier, M. The design of anionic surfactant-based amino-functionalized mesoporous silica nanoparticles and their application in transdermal drug delivery. Pharmaceutics 2020, 12, 1035. [Google Scholar] [CrossRef]
- Cui, L.; Liu, W.; Liu, H.; Qin, Q.; Wu, S.; He, S.; Pang, X.; Zhu, C.; Shen, P. pH-triggered charge-reversal mesoporous silica nanoparticles stabilized by chitosan oligosaccharide/carboxymethyl chitosan hybrids for effective intracellular delivery of doxorubicin. ACS Appl. Bio Mater. 2019, 2, 1907–1919. [Google Scholar] [CrossRef]
- Nasab, N.A.; Kumleh, H.H.; Beygzadeh, M.; Teimourian, S.; Kzemzad, M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Art. Cells Nanomed. Biotechnol. 2018, 46, 75–81. [Google Scholar] [CrossRef]
- Castro, L.E.S.P.W.; Kviecinski, M.R.; Ourique, F.; Parisotto, E.B.; Grinevicius, V.M.A.S.; Correia, J.F.G.; Wilhelm Filho, D.; Pedrosa, R.C. Albendazole as a promising molecule for tumor control. Redox Biol. 2016, 10, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Sawatdee, S.; Atipairin, A.; Sae Yoon, A.; Srichana, T.; Changsan, N.; Suwandecha, T. Formulation Development of Albendazole-Loaded Self-Microemulsifying Chewable Tablets to Enhance Dissolution and Bioavailability. Pharmaceutics 2019, 11, 134. [Google Scholar] [CrossRef]
- Ramesh, Y.; Balasaradhi, K.; Abhilash, K.R. Formulation and evaluation of albendazole nanoparticle. J. Drug Deliv. Ther. 2019, 9, 16–22. [Google Scholar] [CrossRef]
- Noorani, L.; Stenzel, M.; Liang, R.; Pourgholami, M.H.; Morri, D.L. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnol. 2015, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Choi, J.S.; Lee, S.E.; Lee, J.K.; Kim, T.H.; Jang, W.S.; Tunsirikongkon, A.; Kim, J.K.; Park, J.S. Enhancing the in vitro anticancer activity of albendazole incorporated into chitosan-coated PLGA nanoparticles. Carbohydr. Polym. 2017, 159, 39–47. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Guan, Y.; Ding, J.; Ma, C.; Xie, Z. Vaginal drug delivery approaches for localized management of cervical cancer. Adv. Drug Deliv. Rev. 2021, 174, 114–126. [Google Scholar] [CrossRef]
- De Souza Costa, E.; Pereira, M.M.; Mansur, H.S. Properties, and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci. Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef]
- Wadell, W.H.; Evans, L.R. Amorphous Silica, in Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 21, p. 483. [Google Scholar]
- Kumar, S.; Koh, J. Physiochemical, Optical and Biological Activity of Chitosan-Chromone Derivative for Biomedical Applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [Green Version]
- Faheem, M.; Moyle, P.M.; Tan, M.S.A.; Thurecht, K.F.; Falconer, J.R. Preparation of albendazole-loaded liposomes by supercritical carbon dioxide processing. Artif. Cells Nanomed. Biotechnol. 2019, 46, S1186–S1192. [Google Scholar]
- Gedam, A.H.; Dongre, R.S. Adsorption characterization of Pb (II) ions onto iodate doped chitosan composite: Equilibrium and kinetic studies. RSC Adv. 2015, 5, 54188–54201. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Kunc, F.; Balhara, V.; Sun, Y.; Daroszewska, M.; Jakub, Z.J.; Hill, M.; Brinkmann, A.; Johnston, L. Quantification of surface functional groups on silica nanoparticles: Comparison of thermogravimetric analysis and quantitative NMR. Analyst 2019, 144, 5589–5599. [Google Scholar] [CrossRef]
- Cavalcanti, N.T.; Souza, G.D.; Tabosa, M.A.M.; Soares Sobrinho, J.L.; Leal, L.B.; de Santana, D.P. Assay and physicochemical characterization of the antiparasitic albendazole. Braz. J. Pharm. Sci. 2012, 48, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Carneiro-da-Cunha, M.; Cerqueria, M.A.; Souza, B.W.S.; Texeira, J.A.; Vicente, A.A. Influence of concentration ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Del Prado-Audelo, M.L.; Caballero-Floran, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urban-Morlán, Z.; Floran, B.; Cortes, H.; Leyva-Gómez, G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101896. [Google Scholar] [CrossRef]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of Zeta Potential and Particle Size on the Stability of SiO2 Nanospheres as Carrier for Ultrasound Imaging Contrast Agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyec, V.; von Haartman, E.; Karam, D.Ş.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar]
- Schubert, J.; Chanana, M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr Med. Chem. 2019, 25, 4556–4586. [Google Scholar] [CrossRef]
- Noorani, L.; Pourgholami, M.H.; Liang, M.; Morris, D.L.; Stenzel, M. Albendazole loaded albumin nanoparticles for ovarian cancer therapy. Eur. J. Nanomed. 2014, 6, 227–236. [Google Scholar] [CrossRef]
- Filippova, M.; Filippov, V.; Williams, V.M.; Zhang, K.; Kokoza, A.; Bashkirova, S.; Duerksen-Hughes, P. Cellular Levels of Oxidative Stress Affect the Response of Cervical Cancer Cells to Chemotherapeutic Agents. Biomed. Res. Int. 2014, 2014, 574659. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, M.; Meka, A.; Liu, Y.; Zhang, J.; Yang, Y.; Yu, C. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J. Mater. Chem. B 2016, 4, 212–219. [Google Scholar] [CrossRef]







| Sample | Mean Size nm | Zeta Potential mV | SBET m2/g | C from BET Equation | Pore Volume cm3/g | Fractal Dimension 1 D |
|---|---|---|---|---|---|---|
| SiO2 | 461 | −51.3 | 11 | 183 | 0.021 | 2.70 * |
| SiO2-MS | 1116 | −33.7 | 144 | 44 | 0.352 | 2.51 |
| SiO2-MS-ABZ | 1106 | −1.37 | 150 | 37 | 0.384 | 2.50 |
| SiO2-MS-ABZ-CS | 951 | +1.61 | 80 | 26 | 0.367 | 2.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Castillo, D.J.; de la Cruz Hernández, E.N.; Frías Márquez, D.M.; Tilley, R.D.; Gloag, L.; Owen, P.Q.; López González, R.; Alvarez Lemus, M.A. Albendazole Release from Silica-Chitosan Nanospheres. In Vitro Study on Cervix Cancer Cell Lines. Polymers 2021, 13, 1945. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121945
Hernández-Castillo DJ, de la Cruz Hernández EN, Frías Márquez DM, Tilley RD, Gloag L, Owen PQ, López González R, Alvarez Lemus MA. Albendazole Release from Silica-Chitosan Nanospheres. In Vitro Study on Cervix Cancer Cell Lines. Polymers. 2021; 13(12):1945. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121945
Chicago/Turabian StyleHernández-Castillo, Daniela J., Erick Natividad de la Cruz Hernández, Dora M. Frías Márquez, Richard D. Tilley, Lucy Gloag, Patricia Quintana Owen, Rosendo López González, and Mayra A. Alvarez Lemus. 2021. "Albendazole Release from Silica-Chitosan Nanospheres. In Vitro Study on Cervix Cancer Cell Lines" Polymers 13, no. 12: 1945. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13121945