Water-Induced Breaking of Interfacial Cohesiveness in a Poly(lactic acid)/Miscanthus Fibers Biocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Additive Manufacturing of PLA
2.3. Processing of the PLA-Based Biocomposite
2.4. Crystallization and Immersion
2.5. Material Characterization
3. Results and Discussion
3.1. Post-Immersion Properties of the Additive Manufactured PLA
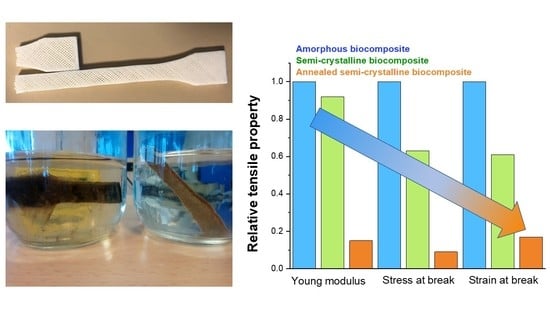
3.2. Post-Immersion Properties of the Biocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Zhuge, Y.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction–A review on global waste generation, performance, application and future opportunities. Resour. Conser. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Singh, A.A.; Genovese, M.E.; Mancini, G.; Marini, L.; Athanassiou, A. Green processing route for polylactic acid–cellulose fiber biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 4128–4136. [Google Scholar] [CrossRef]
- Zhao, X.; Li, K.; Wang, Y.; Tekinalp, H.; Larsen, G.; Rasmussen, D.; Ginder, R.S.; Wang, L.; Gardner, D.J.; Tajvidi, M.; et al. High-strength polylactic acid (PLA) biocomposites reinforced by epoxy-modified pine fibers. ACS Sustain. Chem. Eng. 2020, 8, 13236–13247. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K. Modification of brittle polylactide by novel hyperbranched polymer-based nanostructures. Biomacromolecules 2007, 8, 2476–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super tough poly(lactic acid) blends: A comprehensive review. RSC Adv. 2020, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Karami, S.; Lafleur, P.G. Toughening of polylactide nanocomposites with an ethylene alkyl acrylate copolymer: Effects of the addition of nanoparticles on phase morphology and fracture mechanisms. Polym. Eng. Sci. 2016, 56, 1415–1424. [Google Scholar] [CrossRef]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef] [PubMed]
- Rocca–smith, J.R.; Whyte, O.; Brachais, C.–H.; Champion, D.; Piasente, F.; Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Karbowiak, T. Beyond biodegradability of poly(lactic acid): Physical and chemical stability in humid environments. ACS Sustain. Chem. Eng. 2017, 5, 2751–2762. [Google Scholar] [CrossRef]
- Deroiné, M.; Le Duigou, A.; Corre, Y.–M.; Le Gac, P.–Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated ageing of polylactide in aqueous environments: Comparative study between distilled water and seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Moetazedian, A.; Gleadall, A.; Han, X.; Ekinci, A.; Mele, E.; Silberschmidt, V.V. Mechanical performance of 3D printed polylactide during degradation. Addit. Manuf. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Fernandes Nassar, S.; Guinault, A.; Delpouve, N.; Divry, V.; Ducruet, V.; Sollogoub, C.; Domenek, S. Multi-scale analysis of the impact of polylactide morphology on gas barrier properties. Polymer 2017, 108, 163–172. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic mechanical analysis investigations of PLA-based renewable materials: How are they useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L. Calorimetric analysis of the multiple melting behavior of poly(L-lactic acid). J. Appl. Polym. Sci. 2006, 100, 3145–3151. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E. Crystalline, mobile amorphous and rigid amorphous fractions in poly(L-lactic acid) by TMDSC. Thermochim. Acta 2011, 522, 118–127. [Google Scholar] [CrossRef]
- Saiter, A.; Delpouve, N.; Dargent, E.; Oberhauser, W.; Conzatti, L.; Cicogna, F.; Passaglia, E. Probing the chain segment mobility at the interface of semi-crystalline polylactide/clay nanocomposites. Eur. Polym. J. 2016, 78, 274–289. [Google Scholar] [CrossRef]
- Cocca, M.; Di Lorenzo, M.L.; Malinconico, M.; Frezza, V. Influence of crystal polymorphism on mechanical and barrier properties of poly(L-lactic acid). Eur. Polym. J. 2011, 47, 1073–1080. [Google Scholar] [CrossRef]







| TGA (°C) | MT-DSC | ||||||
|---|---|---|---|---|---|---|---|
| T5% | T10% | T50% | Tmax | Xc | XMAF | XRAF | |
| Amorphous PLA | 316 | 329 | 358 | 366 | 0 | 1 | 0 |
| PLA crystallized at 80 °C | 321 | 333 | 360 | 367 | 0.33 | 0.31 | 0.36 |
| PLA crystallized at 135 °C | 320 | 331 | 359 | 367 | 0.50 | 0.35 | 0.15 |
| Tensile Tests | Charpy | |||
|---|---|---|---|---|
| E (MPa) | σ Break (MPa) | ε Break (%) | Klc (kJ m−2) | |
| Amorphous PLA | 920 | 30 | 4.0 | 16 |
| Amorphous PLA (2 years of immersion) | 850 | 27 | 4.0 | / |
| PLA crystallized at 80 °C | 780 | 20 | 3.5 | 16 |
| PLA crystallized at 80 °C (2 years of immersion) | 880 | 18 | 4.0 | / |
| PLA crystallized at 135 °C | 950 | 6.5 | 1.0 | 4 |
| PLA crystallized at 135 °C (2 years of immersion) | 1000 | 6.0 | 1.0 | / |
| TGA (°C) | MT-DSC | ||||
|---|---|---|---|---|---|
| T5% | T10% | T50% | Tmax | Xc | |
| PLA4043D_EA_FM | 302 | 319 | 359 | 365 | 0.05 |
| PLLA_EA_FM | 305 | 323 | 359 | 365 | 0.35 |
| annealed PLLA_EA_FM | 302 | 319 | 354 | 360 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delpouve, N.; Faraj, H.; Demarest, C.; Dontzoff, E.; Garda, M.-R.; Delbreilh, L.; Berton, B.; Dargent, E. Water-Induced Breaking of Interfacial Cohesiveness in a Poly(lactic acid)/Miscanthus Fibers Biocomposite. Polymers 2021, 13, 2285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142285
Delpouve N, Faraj H, Demarest C, Dontzoff E, Garda M-R, Delbreilh L, Berton B, Dargent E. Water-Induced Breaking of Interfacial Cohesiveness in a Poly(lactic acid)/Miscanthus Fibers Biocomposite. Polymers. 2021; 13(14):2285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142285
Chicago/Turabian StyleDelpouve, Nicolas, Hajar Faraj, Clément Demarest, Eric Dontzoff, Marie-Rose Garda, Laurent Delbreilh, Benjamin Berton, and Eric Dargent. 2021. "Water-Induced Breaking of Interfacial Cohesiveness in a Poly(lactic acid)/Miscanthus Fibers Biocomposite" Polymers 13, no. 14: 2285. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142285