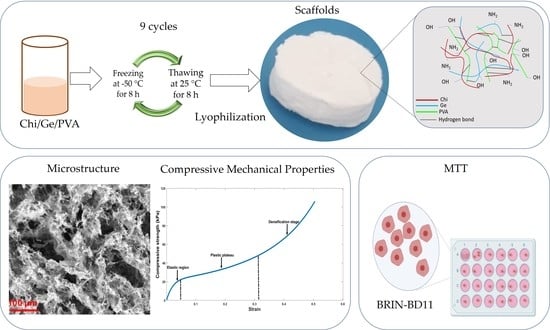
Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan, Gelatin, and PVA Solution
2.3. Preparation of Samples
2.4. Morphological Properties
2.5. Porosity Measurement
2.6. Infrared Spectroscopy
2.7. Swelling Test
2.8. Degradation Test
2.9. Design, Prototyping, and Manufacture of Molds
2.10. Compressive Mechanical Properties
2.11. Cell Culture
2.12. MTT Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Microstructure
3.2. Porosity Measurement
3.3. FTIR Spectra of Samples
3.4. Swelling Capacity
3.5. Degradation
3.6. Compressive Behaviors of the Scaffolds
3.7. Cytocompatibility of Ternary Blend Scaffolds and Controls
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloysious, N.; Nair, P. Enhanced survival and function of islet-like clusters differentiated from adipose stem cells on a three-dimensional natural polymeric scaffold: An in vitro study. Tissue Eng. Part A 2014, 20, 1508–1522. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Nadri, S.; Barati, G.; Mostafavi, H.; Esmaeilzadeh, A.; Enderami, S.E. Differentiation of conjunctiva mesenchymal stem cells into secreting islet beta cells on plasma treated electrospun nanofibrous scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Urrutia, V.A. Efecto de Diferentes Métodos de Entrecruzamiento sobre las Propiedades Fisicoquímicas y Biológicas de Andamios de Quitosano-Colágeno. Ph.D. Thesis, Centro de Investigación científica de Yucatán, Mérida, Mexico, 2019. [Google Scholar]
- Willerth, S.M.; Arendas, K.J.; Gottlieb, D.I.; Sakiyama-Elbert, S.E. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials 2006, 27, 5990–6003. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, R.L.; Sampson, J.P.; Lebioda, K.R.; Shea, L.D. Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters. Acta Biomater. 2019, 96, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Kim, M.S.; Sampattavanich, S.; Baek, J.H.; Zhang, Z.; Elisseeff, J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells 2006, 24, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Genové, E.; Borrós, S.; Semino, C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006, 12, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.Y.; Raghunath, M.; Whitelock, J.; Poole, L. Matrix components and scaffolds for sustained islet function. Tissue Eng. Part B 2011, 17, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.E.; Beenken-Rothkopf, L.N.; Mirsoian, A.; Kojic, N.; Kaplan, D.L.; Barron, A.E.; Fontaine, M.J. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials 2012, 33, 6691–6697. [Google Scholar] [CrossRef] [Green Version]
- Getova, V.E.; van Dongen, J.A.; Brouwer, L.A.; Harmsen, M.C. Adipose tissue-derived ECM hydrogels and their use as 3D culture scaffold. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittes, G.K. Developmental biology of the pancreas: A comprehensive review. Dev. Biol. 2009, 326, 4–35. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef] [PubMed]
- Daoud, J.T.; Petropavlovskaia, M.S.; Patapas, J.M.; Degrandpré, C.E.; DiRaddo, R.W.; Rosenberg, L.; Tabrizian, M. Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials 2011, 32, 1536–1542. [Google Scholar] [CrossRef]
- Skrzypek, K.; Groot Nibbelink, M.; van Lente, J.; Buitinga, M.; Engelse, M.A.; de Koning, J.P.E.; Karperien, M.; van Apeldoorn, A.; Stamatialis, D. Pancreatic islet macroencapsulation using microwell porous membranes. Sci. Rep. 2017, 7, 9186. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, D.G.; Jang, J. 3D pancreatic tissue modeling in vitro: Advances and prospects. Biochip J. 2020, 14, 84–99. [Google Scholar] [CrossRef] [Green Version]
- Kin, T.; O’Neil, J.J.; Pawlick, R.; Korbutt, G.S.; Shapiro, A.M.J.; Lakey, J.R.T. The use of an approved biodegradable polymer scaffold as a solid support system for improvement of islet engraftment. Artif. Organs 2008, 32, 990–993. [Google Scholar] [CrossRef]
- Blomeier, H.; Zhang, X.; Rives, C.; Brissova, M.; Hughes, E.; Baker, M.; Powers, A.C.; Kaufman, D.B.; Shea, L.D.; Lowe, W.L. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation 2006, 82, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Huang, Y.D.; Wei, Z.; Hou, Y.; Xie, W.J.; Huang, R.P.; Song, Y.M.; Lv, H.G.; Song, C.F. Polyglycolic acid-islet grafts improve blood glucose and insulin concentrations in rats with induced diabetes. Transplant. Proc. 2009, 41, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Buitinga, M.; Truckenmüller, R.; Engelse, M.A.; Moroni, L.; Ten Hoopen, H.W.M.; van Blitterswijk, C.A.; de Koning, E.J.P.; van Apeldoorn, A.A.; Karperien, M. Microwell scaffolds for the extrahepatic transplantation of islets of langerhans. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Papenburg, B.J.; Vogelaar, L.; Bolhuis-Versteeg, L.A.M.; Lammertink, R.G.H.; Stamatialis, D.; Wessling, M. One-step fabrication of porous micropatterned scaffolds to control cell behavior. Biomaterials 2007, 28, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Yao, X.; Wang, M.; Zhao, Y.; Lu, Y.; Wang, Z.; Guo, Y. Biomimetic hybrid scaffold of electrospun silk fibroin and pancreatic decellularized extracellular matrix for islet survival. J. Biomater. Sci. Polym. Ed. 2020, 32, 151–165. [Google Scholar] [CrossRef]
- Liu, L.; Tan, J.; Li, B.; Xie, Q.; Sun, J.; Pu, H.; Zhang, L. Construction of functional pancreatic artificial islet tissue composed of fibroblast-modified polylactic-co-glycolic acid membrane and pancreatic stem cells. J. Biomater. Appl. 2017, 32, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, N.; Lin, X.; Tateishi, T.; Chen, G. Three-dimensional cultures of rat pancreatic RIN-5F cells in porous PLGA-collagen hybrid scaffolds. J. Bioact. Compat. Polym. 2009, 24, 25–42. [Google Scholar] [CrossRef]
- Kaviani, M.; Azarpira, N. Insight into microenvironment remodeling in pancreatic endocrine tissue engineering: Biological and biomaterial approaches. Tissue Eng. Regen. Med. 2016, 13, 475–484. [Google Scholar] [CrossRef]
- Marchioli, G.; Van Gurp, L.; Van Krieken, P.P.; Stamatialis, D.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.B.J.; De Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015, 7, 25009. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Velásquez, C.L. Algunos usos del quitosano en sistemas acuosos. Rev. Iberoam. Polím. 2003, 4, 91. [Google Scholar]
- González, J.R. Propiedades Ópticas, Viscoelásticas y Morfológicas de Disoluciones Acuosas de Quitosano y Nanotubos Multipared de Carbono. Master’s Thesis, Universidad de Sonora, Sonora, Mexico, 2015. [Google Scholar]
- Ramos, M.; Zamora, V.; Rodríguez, G.; Sibaja, M.; Madrigal, S.; Lopretti, M. Andamiajes tridimensionales tipo esponjas basados en bioconjugados colágeno-quitosano como potencial biomaterial para aplicaciones en ingeniería de tejidos. Innotec 2012, 7, 43–48. [Google Scholar]
- Cañas, A.I.; Gartner, C. Preparación de soportes de quitosano-gelatina para su aplicación en la ingeniería de tejidos. Rev. Colomb. Mater. 2014, 5, 21–27. [Google Scholar]
- Mao, J.S.; Zhao, L.G.; Yin, Y.J.; Yao, K. Structure and properties of bilayer chitosan-gelatin scaffolds. Biomaterials 2003, 24, 1067–1074. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. Wiley Intersci. 2004, 1638–1646. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, R.; García, Z.Y.; Jiménez, I.; Jiménez, J.A.; Espinosa, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2018, 136, 1–9. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly (α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Yin, H.; Udomsom, S.; Kantawong, F. Fabrication of blended gelatin-polyvinyl alcohol-chitosan scaffold for wound regeneration. Chiang Mai Univ. J. Nat. Sci. 2020, 19, 930–952. [Google Scholar] [CrossRef]
- ASTM D882-09 Standard test method for tensile properties of thin plastic sheeting (D882- 09). In Annual Book of ASTM Standards; American Society for Testing Materials: Philadelphia, PA, USA, 2009.
- Jiang, G.; Li, Q.; Wang, C.; Dong, J.; He, G. Characterization and investigation of the deformation behavior of porous magnesium scaffolds with entangled architectured pore channels. J. Mech. Behav. Biomed. Mater. 2016, 64, 139–150. [Google Scholar] [CrossRef]
- Mantilla, G.; Ángel, A.; Moreno, N. Effects of oleic (18 0) fatty acids on the metabolic state of adipocytes. Salud UIS 2021, 53. [Google Scholar]
- Ashworth, J.C.; Best, S.M.; Cameron, R.E. Quantitative architectural description of tissue engineering scaffolds. Mater. Technol. 2014, 29, 281–295. [Google Scholar] [CrossRef] [Green Version]
- Practical relevance and interpretation of characterization data. In Characterisation and Design of Tissue Scaffolds; Tomlins, P. (Ed.) Elsevier Ltd.: Alpharetta, GA, USA, 2016; pp. 245–268. ISBN 9781782420958. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Suwatwirote, N.; Pripatnanont, P.; Oungbho, K.; Arpornmaeklong, P. Growth and differentiation of mouse osteoblasts on chitosan-collagen sponges. Int. J. Oral Maxillofac. Surg. 2007, 36, 328–337. [Google Scholar] [CrossRef]
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.P. Fundamentals of Tissue Engineering and Regenerative Medicine, 1st ed.; Meyer, U., Meyer, T., Handschel, J., Wiesmann, H.P., Eds.; Springer: Berlin, Germany, 2009; Volume 1, ISBN 978-3-540-77754-0. [Google Scholar]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De La Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Barraza-Garza, G.; De La Rosa, L.A.; Martínez-Martínez, A.; Castillo-Michel, H.; Cotte, M.; Alvarez-Parrilla, E. La microespectroscopía de infrarrojo con transformada de fourier (FTIRM) en el estudio de sistemas biológicos. Rev. Latinoam. Quim. 2013, 41, 125–148. [Google Scholar]
- Ghaderi, J.; Hosseini, S.F.; Keyvani, N.; Gómez-Guillén, M.C. Polymer blending effects on the physicochemical and structural features of the chitosan/poly(vinyl alcohol)/fish gelatin ternary biodegradable films. Food Hydrocoll. 2019, 95, 122–132. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of chitosan-gelatin based hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr. Polym. 2019, 207, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Li, N.; Li, H.Z.; Li, X.J.; Cao, J.M.; Zhang, G.P.; He, D.L. Preparation and characterization of biocomposite chitosan film containing Perilla frutescens (L.) Britt. essential oil. Ind. Crop. Prod. 2018, 112, 660–667. [Google Scholar] [CrossRef]
- Peng, H.T.; Martineau, L.; Shek, P.N. Hydrogel-elastomer composite biomaterials: 3. Effects of gelatin molecular weight and type on the preparation and physical properties of interpenetrating polymer networks. J. Mater. Sci. Mater. Med. 2008, 19, 997–1007. [Google Scholar] [CrossRef]
- Nugraheni, A.D.; Purnawati, D.; Bimo Anugrah, P.; Chotimah, C.; Kusumaatmaja, A.; Triyana, K. Study of thermal degradation of PVA/Chitosan/Gelatin electrospun nanofibers. AIP Conf. Proc. 2016, 1755. [Google Scholar] [CrossRef] [Green Version]
- Arredondo, A.; Patiño, J.F.; Londoño, M.E.; Echeverri, C.E. Matriz a partir de un hidrogel de alcohol polivinílico (PVA) combinada con sulfadiazina de plata con potencial aplicacion en el manejo y control de la sepsis en heridas dérmicas. Rev. Iberoam. Polím. 2011, 12, 178–187. [Google Scholar]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Xiao, L.; Tang, Q. Preparation and characterization of a novel thermosensitive hydrogel based on chitosan and gelatin blends. J. Appl. Polym. Sci. 2009, 113, 400–407. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer composites with high dielectric performance: Interface engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.-J., AlMaadeed, M.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 9780081009741. [Google Scholar]
- Tomić, S.L.; Mićić, M.M.; Dobić, S.N.; Filipović, J.M.; Suljovrujić, E.H. Smart poly(2-hydroxyethyl methacrylate/itaconic acid) hydrogels for biomedical application. Radiat. Phys. Chem. 2010, 79, 643–649. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Abouei Mehrizi, A.; Ghaee, A.; Koosha, M. Electrospinning of PVA/chitosan nanocomposite nanofibers containing gelatin nanoparticles as a dual drug delivery system. J. Biomed. Mater. Res. Part A 2015, 103, 3852–3862. [Google Scholar] [CrossRef] [PubMed]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Resende, C.X.; Tavares, D.S.; Soares, G.A.; Castro, L.O.; Granjeiro, J.M. Cytocompatibility of chitosan and collagen-chitosan scaffolds for tissue engineering. Polimeros 2011, 21, 1–6. [Google Scholar] [CrossRef]
- Dhote, V.; Vernerey, F.J. Mathematical model of the role of degradation on matrix development in hydrogel scaffold. Biomech. Model. Mechanobiol. 2014, 13, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.N.; Barnes, C.A.; Kasick, R.T.; Michel, R.; Gilbert, T.W.; Beer-Stolz, D.; Castner, D.G.; Ratner, B.D.; Badylak, S.F. Surface characterization of extracellular matrix scaffolds. Biomaterials 2010, 31, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleaver, O. Specifying the pancreatic islet through biomechanical forces. N. Engl. J. Med. 2019, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, A.; Prawiro, C.; Seymour, P.A.; de Lichtenberg, K.H.; Jackson, A.; Serup, P.; Semb, H. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 2018, 564, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Organization for Standardization (ISO). Biological Evaluation Of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Liu, Y.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Thermal behavior and mechanical properties of physically crosslinked PVA/Gelatin hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aamodt, K.I.; Powers, A.C. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes. Metab. 2017, 19, 124–136. [Google Scholar] [CrossRef] [Green Version]












| Scaffolds | Blending Ratio | ID | Color |
|---|---|---|---|
| Ternary blend scaffolds | 1:1:1 | P1 |  |
| 2:2:1 | P2 |  | |
| 2:3:1 | P3 |  | |
| 3:2:1 | P4 |  | |
| Control | 1:1:0 | P5 |  |
| 1:0:1 | P6 |  | |
| 0:1:1 | P7 |  | |
| 1:0:0 | P8 |  | |
| 0:1:0 | P9 |  | |
| 0:0:1 | P10 |  |
| Scaffolds | Porosity (%) |
|---|---|
| P1 | 80 ± 0 a,b,c |
| P2 | 90 ± 2.7 a |
| P3 | 85.71 ± 0 a,b |
| P4 | 94.06 ± 0.7 a |
| P5 | 87.85 ± 6.1 a |
| P6 | 83.33 ± 2.4 a,b |
| P7 | 55.56 ± 9.6 c |
| P8 | 91.40 ± 1.5 a |
| P9 | 73.89 ± 6.7 a,b,c |
| P10 | 58.73 ± 13.8 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Cardona, Y.; Echeverri-Cuartas, C.E.; López, M.E.L.; Moreno-Castellanos, N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142372
Sánchez-Cardona Y, Echeverri-Cuartas CE, López MEL, Moreno-Castellanos N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers. 2021; 13(14):2372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142372
Chicago/Turabian StyleSánchez-Cardona, Yesenia, Claudia E. Echeverri-Cuartas, Marta E. Londoño López, and Natalia Moreno-Castellanos. 2021. "Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture" Polymers 13, no. 14: 2372. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13142372