Semi-Fluorinated Di and Triblock Copolymer Nano-Objects Prepared via RAFT Alcoholic Dispersion Polymerization (PISA)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Synthesis of Poly(2-(N,N,dimethylamino)ethyl Methacrylate) (PDMA) Macro-CTA
2.1.2. Synthesis of Poly(2-(N,N,dimethylamino)ethyl Methacrylate)-poly(benzyl methacrylate) (PDMA-PBzMA) Diblock Copolymer Particles via RAFT Dispersion Polymerisation in Ethanol
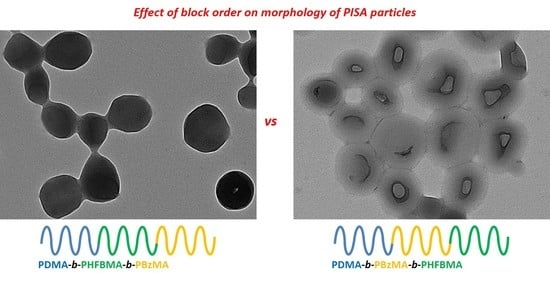
2.1.3. Synthesis of Poly(2-(N,N,dimethylamino)ethyl Methacrylate)-poly(benzyl methacrylate)-poly(heptafluorobutyl methacrylate) (PDMA-PBzMA-PHFBMA) Triblock Copolymer Particles Was Performed via RAFT Dispersion Polymerisation in Ethanol
2.2. Analysis and Characterization of Block Copolymers
3. Results and Discussion
3.1. RAFT Dispersion Polymerization of HFBMA in Ethanol
3.2. PDMA-PBzMA-PTDFOA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Dussert, M.V.; Sousa, N.B.T.; Whittaker, M.R.; Davis, T.P. Reproducible access to tunable morphologies via the self-assembly of an amphiphilic diblock copolymer in water. ACS Macro Lett. 2015, 4, 381–386. [Google Scholar] [CrossRef]
- Semsarilar, M.; Abetz, V. Polymerizations by RAFT: Developments of the Technique and Its Application in the Synthesis of Tailored (Co)polymers. Macromol. Chem. Phys. 2021, 222. [Google Scholar] [CrossRef]
- Lifeng Zhang, A.E. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Chen, Z.; Zhong, S.; Wooley, K.L.; Pochan, D.J. Block copolymer assembly via kinetic control. Science 2007, 317, 647–650. [Google Scholar] [CrossRef]
- Lowe, A.B. RAFT alcoholic dispersion polymerization with polymerization-induced self-assembly. Polymer 2016, 106, 161–181. [Google Scholar] [CrossRef]
- Rieger, J. Guidelines for the Synthesis of Block Copolymer Particles of Various Morphologies by RAFT Dispersion Polymerization. Macromol. Rapid Commun. 2015, 36, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2020, 59, 8368–8392. [Google Scholar] [CrossRef]
- Penfold, N.J.W.; Yeow, J.; Boyer, C.; Armes, S.P. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. [Google Scholar] [CrossRef] [Green Version]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A Critical Appraisal of RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef]
- Mane, S.R. Trending methods employed for polymerization induced self-assembly. New J. Chem. 2020, 44, 6690–6698. [Google Scholar] [CrossRef]
- Semsarilar, M.; Ladmiral, V.; Blanazs, A.; Armes, S.P. Poly(methacrylic acid)-based AB and ABC block copolymer nano-objects prepared via RAFT alcoholic dispersion polymerization. Polym. Chem. 2014, 5. [Google Scholar] [CrossRef]
- Gonzato, C.; Semsarilar, M.; Jones, E.R.; Li, F.; Krooshof, G.J.P.; Wyman, P.; Mykhaylyk, O.O.; Tuinier, R.; Armes, S.P. Rational synthesis of low-polydispersity block copolymer vesicles in concentrated solution via polymerization-induced self-assembly. J. Am. Chem. Soc. 2014, 136. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Jones, E.R.; Blanazs, A.; Armes, S.P. Efficient synthesis of sterically-stabilized nano-objects via RAFT dispersion polymerization of benzyl methacrylate in alcoholic media. Adv. Mater. 2012, 24. [Google Scholar] [CrossRef]
- Jones, E.R.; Semsarilar, M.; Blanazs, A.; Armes, S.P. Efficient synthesis of amine-functional diblock copolymer nanoparticles via RAFT dispersion polymerization of benzyl methacrylate in alcoholic media. Macromolecules 2012, 45. [Google Scholar] [CrossRef]
- Brotherton, E.E.; Hatton, F.L.; Cockram, A.A.; Derry, M.J.; Czajka, A.; Cornel, E.J.; Topham, P.D.; Mykhaylyk, O.O.; Armes, S.P. In Situ Small-Angle X-ray Scattering Studies during Reversible Addition-Fragmentation Chain Transfer Aqueous Emulsion Polymerization. J. Am. Chem. Soc. 2019, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czajka, A.; Armes, S.P. In situ SAXS studies of a prototypical RAFT aqueous dispersion polymerization formulation: Monitoring the evolution in copolymer morphology during. Chem. Sci. 2020, 11, 11443–11454. [Google Scholar] [CrossRef] [PubMed]
- György, C.; Derry, M.J.; Cornel, E.J.; Armes, S.P. Synthesis of Highly Transparent Diblock Copolymer Vesicles via RAFT Dispersion Polymerization of 2,2,2-Trifluoroethyl Methacrylate in n-Alkanes. Macromolecules 2021, 54, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Cornel, E.J.; Van Meurs, S.; Smith, T.; O’Hora, P.S.; Armes, S.P. In Situ Spectroscopic Studies of Highly Transparent Nanoparticle Dispersions Enable Assessment of Trithiocarbonate Chain-End Fidelity during RAFT Dispersion Polymerization in Nonpolar Media. J. Am. Chem. Soc. 2018, 140, 12980–12988. [Google Scholar] [CrossRef] [Green Version]
- Rymaruk, M.J.; Thompson, K.L.; Derry, M.J.; Warren, N.J.; Ratcliffe, L.P.D.; Williams, C.N.; Brown, S.L.; Armes, S.P. Bespoke contrast-matched diblock copolymer nanoparticles enable the rational design of highly transparent Pickering double emulsions. Nanoscale 2016, 8, 14497–14506. [Google Scholar] [CrossRef] [Green Version]
- Semsarilar, M.; Jones, E.R.; Armes, S.P. Comparison of pseudo-living character of RAFT polymerizations conducted under homogeneous and heterogeneous conditions. Polym. Chem. 2014, 5, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly (meth) acrylate: Synthesis and properties. Polymer 2014, 55, 6197–6211. [Google Scholar] [CrossRef] [Green Version]
- Serkhacheva, N.S.; Smirnov, O.I.; Tolkachev, A. V acrylic acid, fl uoroalkyl acrylates and n-butyl. RSC Adv. 2017, 7, 24522–24536. [Google Scholar] [CrossRef] [Green Version]
- Guerre, M.; Lopez, G.; Ameduri, B.; Semsarilar, M.; Ladmiral, V. Solution self-assembly of fluorinated polymers. Polym. Chem. 2021. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef]
- Zhao, W.; Ta, H.T.; Zhang, C.; Whittaker, A.K. Polymerization-Induced Self-Assembly (PISA)—Control over the Morphology of 19F-Containing Polymeric Nano-objects for Cell Uptake and Tracking. Biomacromolecules 2017, 18, 1145–1156. [Google Scholar] [CrossRef]
- Huo, M.; Zhang, Y.; Zeng, M.; Liu, L.; Wei, Y.; Yuan, J. Morphology Evolution of Polymeric Assemblies Regulated with Fluoro-Containing Mesogen in Polymerization-Induced Self-Assembly. Macromolecules 2017, 50, 8192–8201. [Google Scholar] [CrossRef]
- Huo, M.; Wan, Z.; Zeng, M.; Wei, Y.; Yuan, J. Polymerization-induced self-assembly of liquid crystalline ABC triblock copolymers with long solvophilic chains. Polym. Chem. 2018, 9, 3944–3951. [Google Scholar] [CrossRef]
- Huo, M.; Li, D.; Song, G.; Zhang, J.; Wu, D.; Wei, Y.; Yuan, J. Semi-Fluorinated Methacrylates: A Class of Versatile Monomers for Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2018, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Zeng, M.; Li, D.; Liu, L.; Wei, Y.; Yuan, J. Tailoring the Multicompartment Nanostructures of Fluoro-Containing ABC Triblock Terpolymer Assemblies via Polymerization-Induced Self-Assembly. Macromolecules 2017, 50, 8212–8220. [Google Scholar] [CrossRef]
- Semsarilar, M.; Ladmiral, V.; Blanazs, A.; Armes, S.P. Anionic Polyelectrolyte-Stabilized Nanoparticles via RAFT Aqueous Dispersion Polymerization. Langmuir 2011, 28, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P.; Blanazs, A.; Battaglia, G.; Armes, S.P. Facile synthesis of methacrylic ABC triblock copolymer vesicles by RAFT aqueous dispersion polymerization. Macromolecules 2012, 45, 5081–5090. [Google Scholar] [CrossRef]
- Folgado, E.; Guerre, M.; Bijani, C.; Ladmiral, V.; Caminade, A.-M.M.; Ameduri, B.; Ouali, A. Well-defined poly(vinylidene fluoride) (PVDF) based-dendrimers synthesized by click chemistry: Enhanced crystallinity of PVDF and increased hydrophobicity of PVDF films. Polym. Chem. 2016, 7, 5625–5629. [Google Scholar] [CrossRef]
- Folgado, E.; Guerre, M.; Da Costa, A.; Ferri, A.; Addad, A.; Ladmiral, V.; Semsarilar, M. “‘One-pot’” aminolysis/thia-Michaeladdition preparation of well-defined amphiphilic PVDF-b-PEG-b-PVDF triblock copolymers: Self-assembly behaviour in mixed solvents. Polym. Chem. 2020, 11, 401–410. [Google Scholar] [CrossRef]
- Self-assembly, C.; Copolymer, P.V.-T.; Folgado, E.; Mayor, M.; Ladmiral, V. Evaluation of Self-Assembly Pathways to Control Crystallization-Driven Self-Assembly of a Semicrystalline P(VDF-co-HFP)-b-PEG-b-P(VDF-co-HFP) Triblock Copolymer. Molecules 2020, 25, 4033. [Google Scholar]
- Guerre, M.; Semsarilar, M.; Totée, C.; Silly, G.; Améduri, B.; Ladmiral, V. Self-assembly of poly(vinylidene fluoride)-: Block-poly(2-(dimethylamino)ethylmethacrylate) block copolymers prepared by CuAAC click coupling. Polym. Chem. 2017, 8, 5203–5211. [Google Scholar] [CrossRef]









| No. | Target Composition | Conversion% | Calculated DP * | Mean Diamater | Morphology | |
|---|---|---|---|---|---|---|
| DLS (nm) | TEM (nm) | |||||
| 1 | PDMA50 | 71 | 39 | |||
| 2 | PDMA39-PHFBMA50 | 99 | 50 | 27 (0.08) | 16 | Sphere |
| 3 | PDMA39-PHFBMA100 | 99 | 99 | 70 (0.04) | 31 | Sphere |
| 4 | PDMA39-PHFBMA150 | 99 | 149 | 89 (0.07) | 53 | Sphere |
| 5 | PDMA39-PHFBMA200 | 94 | 188 | 125 (0.08) | 65 | Sphere |
| 6 | PDMA39-PHFBMA300 | 99 | 299 | 184 (0.04) | 99 | Sphere |
| 7 | PDMA39-PHFBMA50-PBzMA70 | 99 | 69 | 60 (0.10) | 28 | Sphere |
| 8 | PDMA39-PHFBMA99-PBzMA70 | 99 | 69 | 70 (0.12) | 48 | Sphere |
| 9 | PDMA39-PHFBMA149-PBzMA70 | 99 | 69 | 80 (0.07) | 224 | Sphere |
| 10 | PDMA39-PHFBMA199-PBzMA70 | 94 | 66 | 375 (0.09) | 213 | Sphere |
| 11 | PDMA39-PHFBMA50-PBzMA200 | 99 | 198 | 400 (0.11) | 30 | Sphere |
| 12 | PDMA39-PHFBMA99-PBzMA200 | 99 | 198 | 1190 (0.13) | 43 | Sphere |
| 13 | PDMA39-PHFBMA149-PBzMA200 | 99 | 198 | 1247 (0.09) | 68 | Sphere |
| 14 | PDMA39-PHFBMA199-PBzMA200 | 99 | 198 | 1810 (0.10) | 99 | Sphere |
| 15 | PDMA39-PBzMA60 | 98 | 59 | 40 (0.05) | 26 | Sphere |
| 16 | PDMA39-PBzMA70 | 99 | 69 | 46 (0.02) | 30 | Sphere |
| 17 | PDMA39-PBzMA85 | 99 | 84 | 87 (0.07) | 60 | Sphere/Worm |
| 18 | PDMA39-PBzMA120 | 97 | 117 | 160 (0.07) | 86 | Worms/Vesicle |
| 19 | PDMA39-PBzMA200 | 99 | 198 | 325 (0.04) | 204 | Vesicle |
| 20 | PDMA39-PBzMA69-PHFBMA50 | 99 | 50 | 100 (0.12) | 48 | Sphere |
| 21 | PDMA39-PBzMA69-PHFBMA100 | 99 | 99 | 125 (0.08) | 93 | Sphere |
| 22 | PDMA39-PBzMA69-PHFBMA150 | 99 | 149 | 200 (0.011) | 120 | Sphere |
| 23 | PDMA39-PBzMA69-PHFBMA200 | 98 | 196 | 200 (0.14) | 336 | Sphere |
| 24 | PDMA39-PBzMA99-PHFBMA100 | 65 | 65 | 360 (0.07) | 150 | Vesicle |
| 25 | PDMA39-PBzMA149-PHFBMA150 | 94 | 141 | 860 (0.10) | 230 | Vesicle |
| 26 | PDMA39-PBzMA198-PHFBMA50 | 99 | 50 | 210 (0.06) | 73 | Sphere |
| 27 | PDMA39-PBzMA198-PHFBMA100 | 96 | 96 | 430 (0.14) | 122 | Fused Spheres |
| 28 | PDMA39-PBzMA198-PHFBMA150 | 96 | 144 | 600 (0.11) | 145 | Vesicle |
| 29 | PDMA39-PBzMA198-PHFBMA200 | 94 | 189 | 2200 (0.12) | 304 | Vesicle |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desnos, G.; Rubio, A.; Gomri, C.; Gravelle, M.; Ladmiral, V.; Semsarilar, M. Semi-Fluorinated Di and Triblock Copolymer Nano-Objects Prepared via RAFT Alcoholic Dispersion Polymerization (PISA). Polymers 2021, 13, 2502. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152502
Desnos G, Rubio A, Gomri C, Gravelle M, Ladmiral V, Semsarilar M. Semi-Fluorinated Di and Triblock Copolymer Nano-Objects Prepared via RAFT Alcoholic Dispersion Polymerization (PISA). Polymers. 2021; 13(15):2502. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152502
Chicago/Turabian StyleDesnos, Gregoire, Adrien Rubio, Chaimaa Gomri, Mathias Gravelle, Vincent Ladmiral, and Mona Semsarilar. 2021. "Semi-Fluorinated Di and Triblock Copolymer Nano-Objects Prepared via RAFT Alcoholic Dispersion Polymerization (PISA)" Polymers 13, no. 15: 2502. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13152502