Viscoelastic Behavior of Drug-Loaded Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.2.1. Microscopic Observations
2.2.2. Dynamic Thermo-Mechanical Analysis (DMTA)
2.2.3. Mechanical Testing
2.3. In Vitro Drug Release Procedure and Associated Measurement
3. Results
3.1. The Effect of Flow Rate and Drug Percentage on the Drug Release
3.2. Polymer Properties Evolution during Drug Release
3.2.1. Free Volume Fraction Evolution of PU
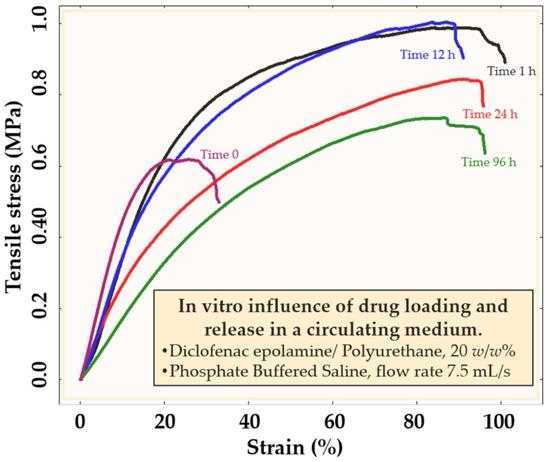
3.2.2. Mechanical Properties of PU Samples
3.2.3. Modeling of the Viscoelastic Behavior by the Cole–Cole Principle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, A.; Farah, S.; Kunduru, K.R.; Doppalapudi, S.; Khan, W.; Domb, A. Polyurethanes for Controlled Drug Delivery; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Wang, W.; Wang, C. Polyurethane for Biomedical Applications: A Review of Recent Developments. Des. Manuf. Med. Devices 2012, 115–151. [Google Scholar] [CrossRef]
- Agnol, L.D.; Dias, F.; Nicoletti, N.F.; Marinowic, D.; Moura e Silva, S.; Marcos-Fernandez, A.; Falavigna, A.; Bianchi, O. Polyurethane tissue adhesives for annulus fibrosus repair: Mechanical restoration and cytotoxicity. J. Biomater. Appl. 2019, 34, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Liou, K.; Jepson, N.; Cao, C.; Luo, R.; Pala, S.; Ooi, S.-Y. Drug-eluting Balloon Versus Second Generation Drug Eluting Stents in the Treatment of In-stent Restenosis: A Systematic Review and Meta-analysis. Heart Lung Circ. 2016, 25, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities Between Peripheral Artery Disease and Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef]
- Adlakha, S.; Sheikh, M.; Wu, J.; Burket, M.W.; Pandya, U.; Colyer, W.; Eltahawy, E.; Cooper, C.J. Stent Fracture in the Coronary and Peripheral Arteries. J. Interv. Cardiol. 2010, 23, 411–419. [Google Scholar] [CrossRef]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraak, R.P.; Hassell, M.E.; Grundeken, M.J.; Koch, K.T.; Henriques, J.P.; Piek, J.J.; Baan, J., Jr.; Vis, M.M.; Arkenbout, E.K.; Tijssen, J.G.; et al. Initial experience and clinical evaluation of the Absorb bioresorbable vascular scaffold (BVS) in real-world practice: The AMC Single Centre Real World PCI Registry. EuroIntervention 2015, 10, 1160–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassese, S.; Byrne, R.; Ndrepepa, G.; Kufner, S.; Wiebe, J.; Repp, J.; Schunkert, H.; Fusaro, M.; Kimura, T.; Kastrati, A. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomised controlled trials. Lancet 2016, 387, 537–544. [Google Scholar] [CrossRef]
- Wang, P.-J.; Ferralis, N.; Conway, C.; Grossman, J.C.; Edelman, E. Strain-induced accelerated asymmetric spatial degradation of polymeric vascular scaffolds. Proc. Natl. Acad. Sci. USA 2018, 115, 2640–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlyle, W.C.; McClain, J.B.; Tzafriri, A.R.; Bailey, L.; Zani, B.G.; Markham, P.M.; Stanley, J.R.; Edelman, E.R. Enhanced drug delivery capabilities from stents coated with absorbable polymer and crystalline drug. J. Control. Release 2012, 162, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Arbeiter, D.; Reske, T.; Teske, M.; Bajer, D.; Senz, V.; Schmitz, K.-P.; Grabow, N.; Oschatz, S. Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices—A Systematic Study. Polymers 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Heljak, M.K.; Chlanda, A.; Swieszkowski, W.; Bil, M. Multiscale analysis of viscoelastic properties, topography and internal structure of a biodegradable thermo-responsive shape memory polyurethane. Polymer 2020, 191, 122273. [Google Scholar] [CrossRef]
- Wrześniewska-Tosik, K.; Ryszkowska, J.; Mik, T.; Wesołowska, E.; Kowalewski, T.; Pałczyńska, M.; Walisiak, D.; Królikowska, M.A.; Leszczyńska, M.; Niezgoda, K.; et al. Viscoelastic Polyurethane Foam with Keratin and Flame-Retardant Additives. Polymers 2021, 13, 1380. [Google Scholar] [CrossRef] [PubMed]
- Belibel, R.; Sali, S.; Marinval, N.; Garcia-Sanchez, A.; Barbaud, C.; Hlawaty, H. PDMMLA derivatives as a promising cardiovascular metallic stent coating: Physicochemical and biological evaluation. Mater. Sci. Eng. C 2020, 117, 111284. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.; Huang, C.L.; Widjaja, E.; Boey, F.Y.; Loo, J.S.; Venkatraman, S.S. The effect of polyethylene glycol structure on paclitaxel drug release and mechanical properties of PLGA thin films. Acta Biomater. 2011, 7, 1973–1983. [Google Scholar] [CrossRef]
- Liu, X.; Lei, L.; Hou, J.-W.; Tang, M.-F.; Guo, S.-R.; Wang, Z.-M.; Chen, K.-M. Evaluation of two polymeric blends (EVA/PLA and EVA/PEG) as coating film materials for paclitaxel-eluting stent application. J. Mater. Sci. Mater. Electron. 2011, 22, 327–337. [Google Scholar] [CrossRef]
- Engineer, C.; Parikh, J.; Raval, A. Effect of copolymer ratio on hydrolytic degradation of poly(lactide-co-glycolide) from drug eluting coronary stents. Chem. Eng. Res. Des. 2011, 89, 328–334. [Google Scholar] [CrossRef]
- Fotaki, N.; Klein, S. In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ansari, M.; Golzar, M.; Baghani, M.; Shirsavar, M.A.; Taghavimehr, M. Force recovery evaluation of thermo-induced shape-memory polymer stent: Material, process and thermo-viscoelastic characterization. Smart Mater. Struct. 2019, 28, 095022. [Google Scholar] [CrossRef]
- Zhu, X.; Braatz, R.D. Modeling and Analysis of Drug-Eluting Stents With Biodegradable PLGA Coating: Consequences on Intravascular Drug Delivery. J. Biomech. Eng. 2014, 136, 111004–11100410. [Google Scholar] [CrossRef] [Green Version]
- Abbasnezhad, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. In vitro study of drug release from various loaded polyurethane samples and subjected to different non-pulsed flow rates. J. Drug Deliv. Sci. Technol. 2020, 55, 101500. [Google Scholar] [CrossRef]
- Wondu, E.; Lule, Z.; Kim, J. Thermal Conductivity and Mechanical Properties of Thermoplastic Polyurethane-/Silane-Modified Al2O3 Composite Fabricated via Melt Compounding. Polymers 2019, 11, 1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Ma, W.; Zhong, J.; Zhang, Z. Comparative Study of Machine Learning Approaches for Predicting Creep Behavior of Polyurethane Elastomer. Polymers 2021, 13, 1768. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: Role of protein–surface interactions. Prog. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Pal, S. Mechanical Properties of Biological Materials. In Design of Artificial Human Joints & Organs; Springer: Boston, MA, USA, 2014. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Kouidri, S.; Salahinejad, E.; Tcharkhtchi, A.; Bakir, F. Controlled release from polyurethane films: Drug release mechanisms. J. Appl. Polym. Sci. 2021, 138, 50083. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; He, Y. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheler, S. The polymer free volume as a controlling factor for drug release from poly(lactide-co-glycolide) microspheres. J. Appl. Polym. Sci. 2013, 131, 1–13. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E.G. Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. On the importance of physical and mechanical properties of PLGA films during drug release. J. Drug Deliv. Sci. Technol. 2021, 63, 102446. [Google Scholar] [CrossRef]
- Bueche, F. Derivation of the WLF Equation for the Mobility of Molecules in Molten Glasses. J. Chem. Phys. 1956, 24, 418–419. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.T. Introduction to Polymer Rheology; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Panwar, V.; Pal, K. An optimal reduction technique for rGO/ABS composites having high-end dynamic properties based on Cole-Cole plot, degree of entanglement and C-factor. Compos. Part B Eng. 2017, 114, 46–57. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic Mechanical Analysis; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Shirinbayan, M.; Montazeri, A.; Sedeh, M.N.; Abbasnezhad, N.; Fitoussi, J.; Tcharkhrtchi, A. Rotational Molding of Polyamide-12 Nanocomposites: Modeling of the Viscoelastic Behavior. Int. J. Mater. Form. 2021, 14, 143–152. [Google Scholar] [CrossRef]
- Quinson, R.; Perez, J.; Germain, Y.; Murraciole, J. β- and α-relaxations in poly(methyl methacrylate) and polycarbonate: Non-linear anelasticity studies by antistress relaxation. Polymer 1995, 36, 743–752. [Google Scholar] [CrossRef]
- Farzaneh, S.; Fitoussi, J.; Lucas, A.; Bocquet, M.; Tcharkhtchi, A. Shape memory effect and properties memory effect of polyurethane. J. Appl. Polym. Sci. 2012, 128, 3240–3249. [Google Scholar] [CrossRef] [Green Version]
- Montazeri, A. The effect of functionalization on the viscoelastic behavior of multi-wall carbon nanotube/epoxy composites. Mater. Des. 2013, 45, 510–517. [Google Scholar] [CrossRef]














| Samples | (MPa) | (MPa) | (s) | |||
|---|---|---|---|---|---|---|
| PU-Pure-0 h | 16 | 2690 | 0.41 | 0.23 | 2 | 0.5 |
| PU-10%-0 h | 14 | 2200 | 0.4 | 0.25 | 1.2 | 1 |
| PU-10%-12 h | 13 | 1715 | 0.37 | 0.24 | 2 | 2 |
| PU-10%-24 h | 12 | 1519 | 0.37 | 0.25 | 2.4 | 3 |
| PU-10%-96 h | 11 | 1100 | 0.45 | 0.3 | 3 | 4 |
| PU-20%-0 h | 14 | 2050 | 0.24 | 0.3 | 2 | 5 |
| PU-20%-12 h | 12 | 1368 | 0.24 | 0.34 | 2 | 6 |
| PU-20%-24 h | 10 | 1180 | 0.24 | 0.34 | 2 | 7 |
| PU-20%-96 h | 7 | 1040 | 0.25 | 0.35 | 2 | 8 |
| Drug Percentage | Parameters of Equation | a | b | c | R2 |
|---|---|---|---|---|---|
| 10% | (MPa) | 10.291 | −3.747 | 0.97 | 0.997 |
| (MPa) | 1086.214 | 1103.039 | 0.959 | 0.996 | |
| (s) | 4.129 | 3.129 | 0.967 | 0.984 | |
| 20% | (MPa) | 5.876 | −8.194 | 0.973 | 0.998 |
| (MPa) | 1048.920 | 998.835 | 0.912 | 0.999 | |
| (s) | 8.051 | 3.051 | 0.958 | 0.991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasnezhad, N.; Shirinbayan, M.; Chabi, F.; Champmartin, S.; Tcharkhtchi, A.; Bakir, F. Viscoelastic Behavior of Drug-Loaded Polyurethane. Polymers 2021, 13, 2608. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162608
Abbasnezhad N, Shirinbayan M, Chabi F, Champmartin S, Tcharkhtchi A, Bakir F. Viscoelastic Behavior of Drug-Loaded Polyurethane. Polymers. 2021; 13(16):2608. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162608
Chicago/Turabian StyleAbbasnezhad, Navideh, Mohammadali Shirinbayan, Fatiha Chabi, Stephane Champmartin, Abbas Tcharkhtchi, and Farid Bakir. 2021. "Viscoelastic Behavior of Drug-Loaded Polyurethane" Polymers 13, no. 16: 2608. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162608