Properties and Degradation Performances of Biodegradable Poly(lactic acid)/Poly(3-hydroxybutyrate) Blends and Keratin Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Preparation and Chemical Characterization of Prepared Keratin Composites
3.2. Thermal Properties of Keratin Composites Inspected by DSC and TGA Analyses
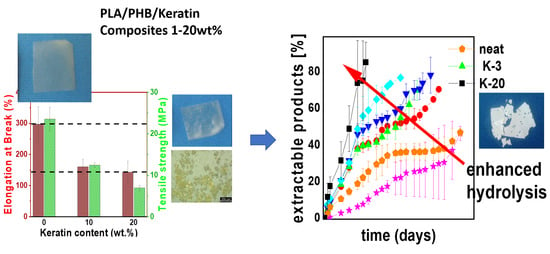
3.3. Mechanical Properties
3.4. Hydrolytic Degradation of Keratin Composites
3.5. Microbial Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Opálková Šišková, A.; Kleinová, A.; Danko, M.; Mosnáček, J. Properties and Degradation of Novel Fully Biodegradable PLA/PHB Blends Filled with Keratin. Int. J. Mol. Sci. 2020, 21, 9678. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly(lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Labrecque, L.V.; Kumar, R.A.; Davé, V.; Gross, R.A.; Mccarthy, S.P. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Gorrasi, G.; Milone, C.; Piperopoulos, E.; Lanza, M.; Sorrentino, A. Hybrid clay mineral-carbon nanotube-PLA nanocomposite films. Preparation and photodegradation effect on their mechanical, thermal and electrical properties. Appl. Clay Sci. 2013, 71, 49–54. [Google Scholar] [CrossRef]
- Nieddu, E.; Mazzucco, L.; Gentile, P.; Benko, T.; Balbo, V.; Mandrile, R.; Ciardelli, G. Preparation and biodegradation of clay composites of PLA. React. Funct. Polym. 2009, 69, 371–379. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Rice husk as bio-source of silica: Preparation and characterization of PLA-silica bio-composites. RSC Adv. 2014, 4, 54703–54712. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Wei, P.; Jiang, P.; Li, Z.; Yan, Y.; Ji, K. Aluminated mesoporous silica as novel high-effective flame retardant in polylactide. Compos. Sci. Technol. 2013, 82, 1–7. [Google Scholar] [CrossRef]
- Wu, J.H.; Yen, M.S.; Kuo, M.C.; Chen, B.H. Physical properties and crystallization behavior of silica particulates reinforced poly(lactic acid) composites. Mater. Chem. Phys. 2013, 142, 726–733. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ma, X.; Fang, J. Influence of carbon black on the properties of plasticized poly(lactic acid) composites. Polym. Degrad. Stab. 2008, 93, 1044–1052. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, B.; Bian, X.; Feng, L.; Zhang, H.; Duan, R.; Sun, J.; Pang, X.; Chen, W.; Chen, X. Synergistic effect of PLA–PBAT–PLA tri-block copolymers with two molecular weights as compatibilizers on the mechanical and rheological properties of PLA/PBAT blends. RSC Adv. 2015, 5, 73842–73849. [Google Scholar] [CrossRef]
- Hassouna, F.; Raquez, J.M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Sirisinha, K.; Somboon, W. Melt characteristics, mechanical, and thermal properties of blown film from modified blends of poly(butylene adipate-co-terephthalate) and poly(lactide). J. Appl. Polym. Sci. 2012, 124, 4986–4992. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending Polylactic Acid with Polyhydroxybutyrate: The Effect on Thermal, Mechanical, and Biodegradation Properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Yuan, J.; Shen, J.; Kang, I.K. Fabrication of protein-doped PLA composite nanofibrous scaffolds for tissue engineering. Polym. Int. 2008, 57, 1188–1193. [Google Scholar] [CrossRef]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2020, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Zoccola, M.; Aluigi, A.; Patrucco, A.; Tonin, C. Extraction, Processing and Applications of Wool Keratin. Keratin: Structure, Properties and Applications; Book Chapter; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 37–62. [Google Scholar]
- Tanasa, C.E.; Spiridon, I. PLA/chitosan/keratin composites for biomedical applications. Mater. Sci. Eng. C 2014, 40, 242–247. [Google Scholar] [CrossRef]
- Bhagabati, P.; Bhasney, S.M.; Bose, D.; Remadevi, R.; Setty, M.; Rajkhowa, R.; Katiyar, V. Silk and Wool Protein Micro-Particle Reinforced Crystalline Polylactic Acid Bio-Composites with Improved Cell Interaction for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 4739–4751. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Ozan Başar, A.; Turkoğlu Şaşmazel, H.; Bulent Ustundag, C.; Sengor, M.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yuan, J.; Shen, J. Differences in cytocompatibility between collagen, gelatin and keratin. Mater. Sci. Eng. C 2016, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Li, Y.; Liu, X.; Zhang, J.; Zhang, Y. Strategy to introduce a hydroxyapatite-keratin nanocomposite into a fibrous membrane for bone tissue engineering. J. Mater. Chem. B 2013, 1, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernandez, C.G.; Velasco-Santos, C.; Hernandez-Zea, A.L.; Gomez-Guzman, O.; Yañez-Limon, J.M.; Rivera-Armenta, J.L.; Martinez-Hernandez, A.L. Low Concentrations for Significant Improvements in Thermal and Thermomechanical Properties of Poly(Lactic Acid)–Keratin Biocomposites Obtained by Extrusion and 3D Printing. J. Nat. Fibers 2020, 1–14. [Google Scholar] [CrossRef]
- Ye, J.-P.; Gong, J.-S.; Su, C.; Liu, Y.-G.; Jiang, M.; Pan, H.; Li, R.Y.; Geng, Y.; Xu, Z.-H.; Shi, J.-S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloid Surf. B 2020, 194, 111158. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.-J. Fully Biodegradable Biocomposites with High Chicken Feather Content. Polymers 2017, 9, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañavate, J.; Aymerich, J.; Garrido, N.; Colom, X.; Macanás, J.; Molins, G.; Álvarez, M.; Carrillo, F. Properties and optimal manufacturing conditions of chicken feathers/poly (lactic acid) biocomposites. J. Compos. Mater. 2016, 50, 1671–1683. [Google Scholar] [CrossRef] [Green Version]
- Fraser, R.D.; MacRae, T.P.; Parry, D.A.; Suzuki, E. Intermediate filaments in alpha-keratins. Proc. Natl. Acad. Sci. USA 1986, 83, 1179–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyašovský, J.; Sedliačik, J.; Šimon, P.; Novák, I.; Krystofiak, T.; Jurkovič, P.; Sedliačiková, M.; Cibulková, Z.; Mičušík, M.; Kleinová, A. Antioxidant Activity of Keratin Hydrolysates Studied by DSC. J. Am. Leather Chem. Assoc. 2019, 114, 20–28. [Google Scholar]
- Mosnáčková, K.; Šlosár, M.; Kollár, J.; Janigová, I.; Šišková, A.; Chmela, Š.; Sikorska, W.; Perďochová, D.; Gálisová, I.; Alexy, P.; et al. Ageing of plasticized poly(lactic acid)/poly(3-hydroxybutyrate)/carbon black mulching films during one season of sweet pepper production. Eur. Polym. J. 2019, 114, 81–89. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Danko, M.; Šišková, A.; Falco, L.M.; Janigová, I.; Chmela, Š.; Vanovčanová, Z.; Omaníková, L.; Chodák, I.; Mosnáček, J. Complex study of the physical properties of a poly(lactic acid)/poly(3-hydroxybutyrate) blend and its carbon black composite during various outdoor and laboratory ageing conditions. RSC Adv. 2017, 7, 47132–47142. [Google Scholar] [CrossRef] [Green Version]
- Mosnáčková, K.; Šišková, A.; Janigová, I.; Kollár, J.; Šlosár, M.; Chmela, Š.; Alexy, P.; Chodák, I.; Bočkaj, J.; Mosnáček, J. Ageing of plasticized poly(lactic acid)/poly(β-hydroxybutyrate) blend films under artificial UV irradiation and under real agricultural conditions during their application as mulches. Chem. Pap. 2016, 70, 1268–1278. [Google Scholar] [CrossRef]
- Matyašovský, J.; Sedliačik, J.; Valachová, K.; Novák, I.; Jurkovič, P.; Duchovič, P.; Mičušík, M.; Kleinová, A.; Šoltés, L. Antioxidant Effects of Keratin Hydrolysates. J. Am. Leather Chem. Assoc. 2017, 112, 319–359. [Google Scholar]
- Mokrejš, P.; Hrnčiřík, J.; Janáčová, D.; Svoboda, P. Processing of Keratin Waste of Meat Industry. Asian J. Chem. 2012, 24, 1489–1494. [Google Scholar]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Špitalský, Z.; Lacík, I.; Lathová, E.; Janigová, I.; Chodák, I. Controlled degradation of polyhydroxybutyrate via alcoholysis with ethylene glycol or glycerol. Polym. Degrad. Stab. 2006, 91, 856–861. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properies for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Gámez-Pérez, J.; Velazquez-Infante, J.C.; Franco-Urquiza, E.; Pages, P.; Carrasco, F.; Santana, O.O.; Maspoch, M.L. Fracture behavior of quenched poly(lactic acid). Express Polym. Lett. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, Dispersibility, and Crystallinity of Poly(hydroxybutyrate)/Poly(l-lactic acid) Blends Studied by FT-IR Microspectroscopy and Differential Scanning Calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Pardo-Ibáñez, P.; Lopez-Rubio, A.; Martínez-Sanz, M.; Cabedo, L.; Lagaron, J.M. Keratin-polyhydroxyalkanoate melt-compounded composites with improved barrier properties of interest in food packaging applications. J. Appl. Polym. Sci. 2014, 131, 39947. [Google Scholar] [CrossRef]
- Adam, J.; Korneliusz, B.A.; Agnieszka, M. Dynamic Mechanical Thermal Analysis of Biocomposites Based on PLA and PHBV-A Comparative Study to PP Counterparts. J. Appl. Polym. Sci. 2013, 130, 3175–3183. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Andrade, C.T. The Role of Graphene on Thermally Induced Shape Memory Properties of Poly(Lactic Acid) Extruded Composites. J. Therm. Anal. Calorim. 2021, 143, 3107–3115. [Google Scholar] [CrossRef]
- Cao, Y.; Ju, Y.; Liao, F.; Jin, X.; Dai, X.; Li, J.; Wang, X. Improving the Flame Retardancy and Mechanical Properties of Poly(Lactic Acid) with a Novel Nanorod-Shaped Hybrid Flame Retardant. RSC Adv. 2016, 6, 14852–14858. [Google Scholar] [CrossRef]
- Rodriguez, E.J.; Marcos, B.; Huneault, M.A. Hydrolysis of polylactide in aqueous media. J. Appl. Polym. Sci. 2016, 133, 44152. [Google Scholar] [CrossRef]
- Borská, K.; Kasák, P.; Danko, M.; Mosnáček, J. Photochemical transformation of diketone dopants in polyester matrices: Effect of dopants concentration and polyester structure on changes in molecular characteristics and hydrolysis of the matrices. Polym. Test. 2020, 91, 106821. [Google Scholar] [CrossRef]
- Sikorska, W.; Musiol, M.; Nowak, B.; Pajak, J.; Labuzek, S.; Kowalczuk, M.; Adamus, G. Degradability of polylactide and its blend with poly[(R,S)-3-hydroxybutyrate] in industrial composting and compost extract. Int. Biodeterior. Biodegrad. 2015, 101, 32–41. [Google Scholar] [CrossRef]
- Agarwal, M.; Koelling, K.W.; Chalmers, J. Characterization of the Degradation of Polylactic Acid Polymer in a Solid Substrate Environment. J. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, L.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Jeszeová, L.; Bauerova-Hlinkova, V.; Barath, P.; Puskarova, A.; Buckova, M.; Krakova, L.; Pangallo, D. Biochemical and proteomic characterization of the extracellular enzymatic preparate of Exiguobacterium undae, suitable for efficient animal glue removal. Appl. Microbiol. Biotechnol. 2018, 102, 6525–6536. [Google Scholar] [CrossRef] [PubMed]









| Sample | Tg (°C) | Tcc (°C) | TmPLA (°C) | TmPHB (°C) | ΔHm1 a (J g−1) | ΔHm2 b (J g−1) | χc c (%) |
|---|---|---|---|---|---|---|---|
| PLA/PHB | 54.1 | 123.8 | 152.6 | 173.3 | 47.69 | 44.99 | 47.20 |
| PLA/PHB/ATBC | 35.7 | 94.9 | 147.9 | 163.3 | 47.66 | 42.01 | 47.17 |
| PLA/PHB/ATBC/K-1 | 36.8 | 98.3 | 147.6 | 165.6 | 37.59 | 36.64 | 36.26 |
| PLA/PHB/ATBC/K-3 | 28.5 | 96.9 | 147.4 | 162.6 | 45.11 | 45.95 | 44.65 |
| PLA/PHB/ATBC/K-5 | 31.7 | 98.2 | 147.3 | 163.3 | 33.74 | 34.13 | 33.39 |
| PLA/PHB/ATBC/K-10 | 31.3 | 96.9 | 147.2 | 161.6 | 38.35 | 40.63 | 37.96 |
| PLA/PHB/ATBC/K-20 | 30.1 | 93.4 | 147.1 | 166.3 | 32.56 | 35.88 | 32.23 |
| Sample | Tonset (°C) | TPHB (°C) | TPLA (°C) | R600 °C (%) |
|---|---|---|---|---|
| PLA | 448.7 | - | 472.9 | 0 |
| PHB | 278.9 | 291.7 | - | 0 |
| keratin | 266.2 | - | - | 19.93 |
| PLA/PHB | 265.8 | 283.8 | 359.3 | 0 |
| PLA/PHB/ATBC | 276.3 | 287.2 | 364.9 | 0 |
| PLA/PHB/ATBC/K-1 | 281.7 | 298.5 | 365.4 | 0 |
| PLA/PHB/ATBC/K-3 | 284.3 | 298.9 | 364.6 | 0.93 |
| PLA/PHB/ATBC/K-5 | 286.0 | 299.7 | 364.7 | 0.56 |
| PLA/PHB/ATBC/K-10 | 285.2 | 300.7 | 361.6 | 3.35 |
| PLA/PHB/ATBC/K-20 | 287.5 | 300.6 | 353.8 | 4.96 |
| Sample | Before Hydrolysis | 14 Days | End of Hydrolysis | ||||
|---|---|---|---|---|---|---|---|
| Mn, Mw, (Ð) a g mol−1 | PLA/ PHB b | Mn, Mw, (Ð) a g mol−1 | EH c % | Mn, Mw, (Ð) a g mol−1 | PLA/ PHB b | EH c % | |
| PLA/PHB | 51,200, 115,000 (2.25) | 84/16 | 47,600, 126,700 (2.66) | 8 | 10,200, 58,300 (5.69) | 81/19 | 48 |
| PLA/PHB/ATBC | 45,400, 93,100 (2.05) | 88/12 | 45,800, 109,600 (2.39) | 22 | 19,500, 72,600 (3.73) | 85/15 | 48 |
| PLA/PHB/ATBC/K-1 | 45,400, 89,300 (1.97) | 88/12 | 43,500, 92,400 (2.12) | 40 | 6600, 48,300 (7.36) | 84/16 | 70 |
| PLA/PHB/ATBC/K-3 | 44,000, 89,400 (2.03) | 87/13 | 39,200, 83,700 (2.13) | 40 | 16,200, 63,700 (3.93) d | 82/18 | 70 |
| PLA/PHB/ATBC/K-5 | 38,500, 82,300 (2.14) | 88/12 | 38,800, 94,900 (2.44) | 49 | 6100, 66,300 (10.8) d | 78/22 | 78 |
| PLA/PHB/ATBC/K-10 | 37,800, 80,300 (2.12) | 88/12 | 32,400, 89,000 (2.74) | 55 | 5300, 42,600 (8.04) d | 75/25 | 77 |
| PLA/PHB/ATBC/K-20 | 32,900, 76,900 (2.34) | 88/12 | 29,700, 62,600 (2.11) | 78 | 16,200, 50,900 (3.14) d | 67/33 | 86 |
| Sample | Concentration of Adherent Bacteria in Suspension (CFU/mL) |
|---|---|
| Soil suspension without polymers | 3.00 × 106 |
| PLA/PHB/ATBC | 5.10 × 109 |
| PLA/PHB/ATBC/K-1 | 8.51 × 108 |
| PLA/PHB/ATBC/K-5 | 7.50 × 108 |
| PLA/PHB/ATBC/K-10 | 5.00 × 108 |
| PLA/PHB/ATBC/K-20 | 4.00 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danko, M.; Mosnáčková, K.; Vykydalová, A.; Kleinová, A.; Puškárová, A.; Pangallo, D.; Bujdoš, M.; Mosnáček, J. Properties and Degradation Performances of Biodegradable Poly(lactic acid)/Poly(3-hydroxybutyrate) Blends and Keratin Composites. Polymers 2021, 13, 2693. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162693
Danko M, Mosnáčková K, Vykydalová A, Kleinová A, Puškárová A, Pangallo D, Bujdoš M, Mosnáček J. Properties and Degradation Performances of Biodegradable Poly(lactic acid)/Poly(3-hydroxybutyrate) Blends and Keratin Composites. Polymers. 2021; 13(16):2693. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162693
Chicago/Turabian StyleDanko, Martin, Katarína Mosnáčková, Anna Vykydalová, Angela Kleinová, Andrea Puškárová, Domenico Pangallo, Marek Bujdoš, and Jaroslav Mosnáček. 2021. "Properties and Degradation Performances of Biodegradable Poly(lactic acid)/Poly(3-hydroxybutyrate) Blends and Keratin Composites" Polymers 13, no. 16: 2693. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162693