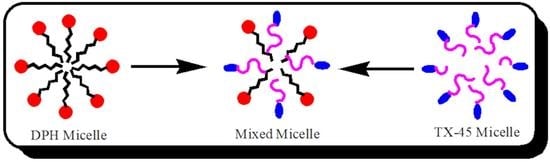
Mixed Micellization and Spectroscopic Studies of Anti-Allergic Drug and Non-Ionic Surfactant in the Presence of Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Tension Measurements
2.2. UV-Visible Spectroscopy
3. Results and Discussion
3.1. Determination of Critical Micelle Concentration (CMC)
3.2. Clint Theory of Mixed Micellization
3.3. Rubingh Theory of Mixed Micellization
3.4. Interfacial Properties of Drug/Surfactant Mixture
3.5. Morphology of Drug/Surfactant Mixture
3.6. Thermodynamics of the Drug/Surfactant Mixture
3.7. UV-Visible Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, T.M.; Hansen, C.B.; de Menezes, D.E.L. Pharmacokinetics of long-circulating liposomes. Adv. Drug Deliv. Rev. 1995, 16, 267–284. [Google Scholar] [CrossRef]
- Canto, G.S.; Dalmora, S.L.; Oliveira, A.G. Piroxicam encapsulated in liposomes: Characterization and in vivo evaluation of topical anti-inflammatory effect. Drug Dev. Ind. Pharm. 1999, 25, 1235–1239. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.-C.; Leroux, J.-C. Polymeric micelles—A new generation of colloidal drug carriers. Eur. J. Pharm. Biopharm. 1999, 48, 101–111. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Trubetskoy, V.S.; Whiteman, K.R.; Ferruti, P.; Veronese, F.M.; Caliceti, P. New synthetic amphiphilic polymers for steric protection of liposomes in vivo. J. Pharm. Sci. 1995, 84, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, S.; Chen, T.; Lin, S.; Jin, H. Micelle formation and drug release behavior of polypeptide graft copolymer and its mixture with polypeptide block copolymer. Int. J. Pharm. 2007, 336, 49–57. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Controll. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Abildskov, J.; O’Connell, J.P. Molecular thermodynamic modeling and design of microencapsulation systems for drug delivery. J. Chem. Eng. Data 2011, 56, 1229–1237. [Google Scholar] [CrossRef]
- Noori, S.; Naqvi, A.Z.; Ansari, W.H.; Kabir-ud-Din. Experimental and theoretical approach to cationic drug-anionic gemini surfactant systems in aqueous medium. Colloids Surf. B Biointerfaces 2014, 115, 71–78. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.; Vieira, D.; Lincopan, N. Cationic surfactants and lipids as anti-infective agents. Anti Infect. Agents Med. Chem. 2006, 5, 33–51. [Google Scholar] [CrossRef]
- Khan, A.M.; Shah, S.S. A UV-visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J. Dispers. Sci. Technol. 2008, 29, 1401–1407. [Google Scholar] [CrossRef]
- Taboada, P.; Ruso, J.M.; Garcia, M.; Mosquera, V. Surface properties of some amphiphilic antidepressant drugs. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 125–128. [Google Scholar] [CrossRef]
- Schreier, S.; Malheiros, S.V.P.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef]
- Naqvi, A.Z.; Noori, S.; Kabir-ud-Din, K.-D. Mixed micellization of dimeric surfactant-amphiphilic drug systems: Effect of surfactant structure. RSC Adv. 2016, 6, 20324–20336. [Google Scholar] [CrossRef]
- Trawiſska, A.; Hallmann, E.; MĿdrzycka, K. The effect of alkyl chain length on synergistic effects in micellization and surface tension reduction in nonionic gemini (S-10) and anionic surfactants mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 114–126. [Google Scholar] [CrossRef]
- Malik, N.A. Solubilization and interaction studies of bile salts with surfactants and drugs: A review. Appl. Biochem. Biotechnol. 2016, 179, 179–201. [Google Scholar] [CrossRef]
- Turovsky, T.; Portnaya, I.; Kesselman, E.; Ionita-Abutbul, I.; Dan, N.; Danino, D. Effect of temperature and loading on the structure of β-casein/ibuprofen assemblies. J. Colloid Interface Sci. 2015, 449, 514–521. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, L. Effects of X-shaped reduction-sensitive amphiphilic block copolymer on drug delivery. Int. J. Nanomed. 2015, 10, 5309. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. Interactions of new 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) based surface active ionic liquids with amitriptyline hydrochloride: Micellization and interfacial studies. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 96–104. [Google Scholar] [CrossRef]
- Srivastava, A.; Thapa, U.; Saha, M.; Jalees, M. Aggregation behaviour of tetracaine hydrochloride with Gemini surfactants and the formation of silver nanoparticles using drug-Gemini surfactants mixture. J. Mol. Liq. 2019, 276, 399–408. [Google Scholar] [CrossRef]
- Saha, U.; Banerjee, A.; Das, B. Drug-surfactant comicellization: Propranolol hydrochloride-surface active ionic liquid systems in aqueous medium. J. Mol. Liq. 2020, 309, 113164. [Google Scholar] [CrossRef]
- Ghasemi, A.; Bagheri, A. Effects of alkyl chain length on synergetic interaction and micelle formation between a homologous series of n-alkyltrimethylammonium bromides and amphiphilic drug propranolol hydrochloride. J. Mol. Liq. 2020, 298, 111948. [Google Scholar] [CrossRef]
- Azum, N.; Ahmed, A.; Rub, M.A.; Asiri, A.M.; Alamery, S.F. Investigation of aggregation behavior of ibuprofen sodium drug under the influence of gelatin protein and salt. J. Mol. Liq. 2019, 290, 111187. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: A tensiometry and fluorometry study. J. Chem. Thermodynam. 2018, 121, 199–210. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 2018, 262, 86–96. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of antipsychotic drug with novel surfactants: Micellization and binding studies. Chin. J. Chem. Eng. 2018, 26, 566–573. [Google Scholar] [CrossRef]
- Khan, F.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: Micellar and thermodynamic investigation. J. Phys. Org. Chem. 2018, 31, e3812. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A.; Azum, N.; Asiri, A.M. Mixed micellization study of ibuprofen (sodium salt) and cationic surfactant (conventional as well as gemini). J. Phys. Org. Chem. 2018, 31, e3730. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Bawazeer, W.A. Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems: Surface tension, fluorescence and UV–vis studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 183–192. [Google Scholar] [CrossRef]
- Azum, N.; Naqvi, A.Z.; Rub, M.A.; Asiri, A.M. Multi-technique approach towards amphiphilic drug-surfactant interaction: A physicochemical study. J. Mol. Liq. 2017, 240, 189–195. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Asiri, A.M. Binary Mixtures of Sodium Salt of Ibuprofen and Selected Bile Salts: Interface, Micellar, Thermodynamic, and Spectroscopic Study. J. Chem. Eng. Data 2017, 62, 3216–3228. [Google Scholar] [CrossRef]
- Rub, M.A.; Azum, N.; Khan, F.; Asiri, A.M. Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J. Phys. Org. Chem. 2017, 30, e3676. [Google Scholar] [CrossRef]
- Raj, S. Simultaneous determination of pseudoephedrine hydrochloride and diphenhydramine hydrochloride in cough syrup by gas chromatography (GC). Talanta 1998, 46, 221–225. [Google Scholar] [CrossRef]
- Raphael, G.D.; Angello, J.T.; Wu, M.-M.; Druce, H.M. Efficacy of diphenhydramine vs desloratadine and placebo in patients with moderate-to-severe seasonal allergic rhinitis. Ann. Allergy Asthma Immunol. 2006, 96, 606–614. [Google Scholar] [CrossRef]
- Taboada, P.; Attwood, D.; Ruso, J.M.; Sarmiento, F.; Mosquera, V. Self-association of amphiphilic penicillins in aqueous electrolyte solution: A light-scattering and NMR study. Langmuir 1999, 15, 2022–2028. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids—Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Rogers, R.D. Room-temperature ionic liquids: New solvents for f -element separations and associated solution chemistry. J. Solid State Chem. 2003, 171, 109–113. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. Immobilized ionic liquids as high-selectivity/high-temperature/high-stability gas chromatography stationary phases. Anal. Chem. 2005, 77, 6453–6462. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Xu, L.; Xiao, J. Ionic liquid-promoted, highly regioselective heck arylation of electron-rich olefins by Aryl Halides. J. Am. Chem. Soc. 2005, 127, 751–760. [Google Scholar] [CrossRef]
- Boxall, D.L.; Osteryoung, R.A. Switching potentials and conductivity of polypyrrole films prepared in the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Electrochem. Soc. 2004, 151, E41. [Google Scholar] [CrossRef]
- Scurto, A.M.; Aki, S.N.V.K.; Brennecke, J.F. Carbon dioxide induced separation of ionic liquids and water. Chem. Commun. 2003, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Kubisa, P. Ionic liquids in the synthesis and modification of polymers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4675–4683. [Google Scholar] [CrossRef]
- Chiappe, C.; Pieraccini, D.; Saullo, P. Nucleophilic displacement reactions in ionic liquids: Substrate and solvent effect in the reaction of NaN 3 and KCN with alkyl halides and tosylates. J. Org. Chem. 2003, 68, 6710–6715. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Kimizuka, N. Interfacial synthesis of hollow TiO2 microspheres in ionic liquids. J. Am. Chem. Soc. 2003, 125, 6386–6387. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Mahajan, S.; Sharma, R.; Mahajan, R.K. An Investigation of drug binding ability of a surface active ionic liquid: Micellization, electrochemical, and spectroscopic studies. Langmuir 2012, 28, 17238–17246. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Rao, K.; Singh, T.; Trivedi, T.J.; Kumar, A. Aggregation behavior of amino acid ionic liquid surfactants in aqueous media. J. Phys. Chem. B 2011, 115, 13847–13853. [Google Scholar] [CrossRef] [PubMed]
- Blesic, M.; Marques, M.H.; Plechkova, N.V.; Seddon, K.R.; Rebelo, L.P.N.; Lopes, A. Self-aggregation of ionic liquids: Micelle formation in aqueous solution. Green Chem. 2007, 9, 481. [Google Scholar] [CrossRef]
- Jiao, J.; Han, B.; Lin, M.; Cheng, N.; Yu, L.; Liu, M. Salt-free catanionic surface active ionic liquids 1-alkyl-3-methylimidazolium alkylsulfate: Aggregation behavior in aqueous solution. J. Colloid Interface Sci. 2013, 412, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Pillania, A. Modulations in surface and aggregation properties of non-ionic surfactant Triton X-45 on addition of ionic liquids in aqueous media. J. Mol. Liq. 2017, 233, 243–250. [Google Scholar] [CrossRef]
- Attwood, D. Micelle formation by some antihistamines in aqueous solution. J. Pharm. Pharmacol. 1972, 24, 751–752. [Google Scholar] [CrossRef]
- Rajput, S.M.; Kumar, S.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I.; Kailasa, S.K. Drug-induced micelle-to-vesicle transition of a cationic gemini surfactant: Potential applications in drug delivery. ChemPhysChem 2018, 19, 865–872. [Google Scholar] [CrossRef]
- Pal, A.; Pillania, A. The effect of hydrophilic ionic liquid 1-butyl-2,3-dimethylimidazolium bromide on the aggregation behavior of tetradecyltrimethylammonium bromide in aqueous media. J. Mol. Liq. 2015, 209, 6–13. [Google Scholar] [CrossRef]
- Behera, K.; Pandey, M.D.; Porel, M.; Pandey, S. Unique role of hydrophilic ionic liquid in modifying properties of aqueous Triton X-100. J. Chem. Phys. 2007, 127, 184501. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, R.K. An investigation of binding ability of ionic surfactants with trifluoperazine dihydrochloride: Insights from surface tension, electronic absorption and fluorescence measurements. RSC Adv. 2012, 2, 9571. [Google Scholar] [CrossRef]
- Motomura, K.; Yamanaka, M.; Aratono, M. Thermodynamic consideration of the mixed micelle of surfactants. Colloid Polym. Sci. 1984, 262, 948–955. [Google Scholar] [CrossRef]
- More, U.; Vaid, Z.; Bhamoria, P.; Kumar, A.; Malek, N.I. Effect of [C n mim][Br] based ionic liquids on the aggregation behavior of tetradecyltrimethylammonium bromide in aqueous medium. J. Solut. Chem. 2015, 44, 850–874. [Google Scholar] [CrossRef]
- Javadian, S.; Ruhi, V.; Asadzadeh Shahir, A.; Heydari, A.; Akbari, J. Imidazolium-based ionic liquids as modulators of physicochemical properties and nanostructures of CTAB in aqueous solution: The effect of alkyl chain length, hydrogen bonding capacity, and anion type. Ind. Eng. Chem. Res. 2013, 52, 15838–15846. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 1976, 72, 1525. [Google Scholar] [CrossRef]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes; John Wiley & Sons.: Hoboken, NJ, USA, 1980; ISBN 0471048933. [Google Scholar]
- Myers, D. Surfactant Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0471746061. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011; ISBN 0080923631. [Google Scholar]
- Tsubone, K.; Arakawa, Y.; Rosen, M.J. Structural effects on surface and micellar properties of alkanediyl-α,ω-bis(sodium N-acyl-β-alaninate) gemini surfactants. J. Colloid Interface Sci. 2003, 262, 516–524. [Google Scholar] [CrossRef]
- Maeda, H. A simple thermodynamic analysis of the stability of ionic/nonionic mixed micelles. J. Colloid Interface Sci. 1995, 172, 98–105. [Google Scholar] [CrossRef]
- Khan, F.; Sheikh, M.S.; Rub, M.A.; Azum, N.; Asiri, A.M. Antidepressant drug amitriptyline hydrochloride (AMT) interaction with anionic surfactant sodium dodecyl sulfate in aqueous/brine/urea solutions at different temperatures. J. Mol. Liq. 2016, 222, 1020–1030. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Self-association and micro-environmental properties of sodium salt of ibuprofen with BRIJ-56 under the influence of aqueous/urea solution. J. Dispers. Sci. Technol. 2017, 38, 96–104. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of triblock-copolymer with cationic gemini and conventional surfactants: A physicochemical study. J. Dispers. Sci. Technol. 2017, 38, 1785–1791. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Bile salt–bile salt interaction in mixed monolayer and mixed micelle formation. J. Chem. Thermodynam. 2019, 128, 406–414. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M.; Kashmery, H.A. Synergistic effect of an antipsychotic drug chlorpromazine hydrochloride with pluronic triblock copolymer: A physicochemical study. J. Mol. Liq. 2018, 260, 159–165. [Google Scholar] [CrossRef]
- Oida, T.; Nakashima, N.; Nagadome, S.; Ko, J.S.; Oh, S.W.; Sugihara, G. Adsorption and micelle formation of mixed surfactant systems in water. III. A comparison between cationic gemini/cationic and cationic gemini/nonionic combinations. J. Oleo Sci. 2003, 52, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]




| α1 | CMC (mM) | CMC* (mM) | X1 | −β | f1 | f2 | ||
|---|---|---|---|---|---|---|---|---|
| TX–45 + DPH | ||||||||
| 0.0 | 112 | −4.89 | ||||||
| 0.1 | 0.69 | 8.39 | 0.59 | 0.93 | 12.45 | 0.128 | 0.012 | |
| 0.3 | 0.49 | 2.94 | 0.65 | 0.98 | 11.41 | 0.249 | 0.008 | |
| 0.5 | 0.39 | 1.79 | 0.68 | 0.99 | 11.36 | 0.317 | 0.005 | |
| 0.7 | 0.23 | 1.28 | 0.69 | 0.99 | 14.09 | 0.258 | 0.001 | |
| 0.9 | 0.21 | 0.99 | 0.71 | 0.99 | 14.72 | 0.295 | 0.001 | |
| 1.0 | 0.90 | |||||||
| TX–45 + DPH + 25 mM IL | ||||||||
| 0 | 93.50 | −7.71 | ||||||
| 0.5 | 0.05 | 0.08 | 0.82 | 0.99 | 9.66 | 0.736 | 0.001 | |
| 1.0 | 0.04 | |||||||
| α1 | 106 Γmax (mol m−2) | Amin (nm2) | pC20 | γCMC (mN m−1) | πCMC (mN m−1) |
|---|---|---|---|---|---|
| TX–45 + DPH | |||||
| 0.0 | 1.809 | 0.917 | 0.940 | 49.76 | 20.24 |
| 0.1 | 1.056 | 1.571 | 3.324 | 48.07 | 21.93 |
| 0.3 | 1.318 | 1.259 | 3.367 | 42.81 | 27.19 |
| 0.5 | 1.279 | 1.297 | 3.961 | 36.94 | 33.06 |
| 0.7 | 0.879 | 1.887 | 4.428 | 34.71 | 35.29 |
| 0.9 | 0.955 | 1.738 | 4.591 | 32.69 | 37.31 |
| 1.0 | 3.827 | 0.433 | 4.910 | 27.49 | 42.51 |
| TX–45 + DPH + 25 mM IL | |||||
| 0 | 1.256 | 1.321 | 0.956 | 50.27 | 19.73 |
| 0.5 | 1.315 | 1.262 | 5.143 | 32.23 | 37.77 |
| 1.0 | 3.249 | 0.510 | 5.511 | 28.31 | 41.69 |
| α1 | P | −B1 | −ΔGM (kJ mol−1) | −ΔGads (kJ mol−1) | Gmin (kJ mol−1) | −Gex | |
|---|---|---|---|---|---|---|---|
| TX–45 + DPH | |||||||
| 0.0 | 0.23 | 15.20 | 26.38 | 27.49 | |||
| 0.1 | 0.13 | 7.63 | 29.92 | 27.99 | 48.75 | 45.49 | 7.44 |
| 0.3 | 0.17 | 6.59 | 29.58 | 28.85 | 49.47 | 32.46 | 6.42 |
| 0.5 | 0.16 | 6.54 | 29.63 | 29.40 | 55.23 | 28.85 | 6.10 |
| 0.7 | 0.11 | 9.27 | 31.09 | 30.71 | 70.82 | 39.44 | 7.46 |
| 0.9 | 0.12 | 9.90 | 31.36 | 30.93 | 69.99 | 34.22 | 7.47 |
| 1.0 | 0.49 | 27.32 | 38.43 | 7.18 | |||
| TX–45 + DPH + 25 mM IL | |||||||
| 0 | 0.16 | 15.82 | 31.52 | 40.00 | |||
| 0.5 | 0.17 | 1.95 | 35.02 | 34.44 | 63.15 | 24.49 | 3.50 |
| 1.0 | 0.41 | 34.92 | 47.75 | 8.71 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azum, N.; Rub, M.A.; Asiri, A.M. Mixed Micellization and Spectroscopic Studies of Anti-Allergic Drug and Non-Ionic Surfactant in the Presence of Ionic Liquid. Polymers 2021, 13, 2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162756
Azum N, Rub MA, Asiri AM. Mixed Micellization and Spectroscopic Studies of Anti-Allergic Drug and Non-Ionic Surfactant in the Presence of Ionic Liquid. Polymers. 2021; 13(16):2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162756
Chicago/Turabian StyleAzum, Naved, Malik Abdul Rub, and Abdullah M. Asiri. 2021. "Mixed Micellization and Spectroscopic Studies of Anti-Allergic Drug and Non-Ionic Surfactant in the Presence of Ionic Liquid" Polymers 13, no. 16: 2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13162756