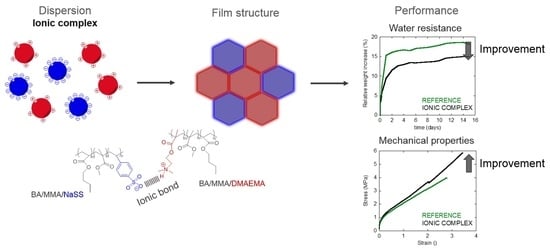
Ionic Inter-Particle Complexation Effect on the Performance of Waterborne Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Computational Details
2.3. Synthesis of Waterborne Polymer Latexes
2.4. Latex Characterization
2.5. Films Characterization
2.6. Blends Preparation and Film Formation
2.7. Blends Preparation and Film Formation for Dye Latexes
3. Results and Discussion
3.1. Theoretical Calculations
3.2. Characteristics of Anionically and Cationically Charged Latexes
3.3. Performance of the Polymer Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paints & Coatings Market Size, Share & Trends Analysis Report By Product (Powdered, Solvent-Borne), By Material (Acrylic, Epoxy), By Application (Architectural & Decorative, Non Architectural), And Segment Forecasts, 2020–2027; Grand View Research. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/paints-coatings-market (accessed on 14 September 2021).
- Paints & Coatings Market: By Resin (Acrylic, Alkyd, Epoxy, Polyurethane, Polyester), Technology (Waterborne, Solventborne, Powder), Application (Architectural [Residential, Non-Residential], Industrial), and Region.—Global Forecasts to 2024; Markets and Markets. 2020. Available online: https://www.researchandmarkets.com/reports/4832924/paints-and-coatings-market-by-resin-acrylic (accessed on 14 September 2021).
- European Commission. Screening Study to Identify Reductions in VOC Emissions due to the Restrictions in the VOC Content of Products; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Directive 2004/42/CE of The European Parliament and of The Council; The European Parliament: Strasbourg, France, 2004.
- Sherman, J.; Chin, B.; Huibers, P.D.T.; Garcia-Valls, R.; Hatton, T.A. Solvent replacement for green processing. Environ. Health Perspect. 1998, 106, 253–271. [Google Scholar]
- Jackson, K. Recent advances in water-borne protective coatings. Surf. Coat. Int. 1999, 7, 340–343. [Google Scholar] [CrossRef]
- Water-Borne vs. Solvent-Borne: “The Gap Continues to Close for Most Applications”. Available online: https://www.european-coatings.com/articles/archiv/water_borne-vs-solvent_borne-the-gap-continues-to-close-for-most-applications (accessed on 14 September 2021).
- Keddie, J.L.; Routh, A.F. Fundamental of Latex Film Formation; Springer Laboratory: Berlin/Heidelberg, Germany, 2010; ISBN 9789048128440. [Google Scholar]
- Keddie, J.L. Film formation of latex. Mater. Sci. Eng. 1997, 21, 101–170. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Liu, Y.; Tomba, J.P.; Soleimani, M.; Lau, W.; Winnik, M.A. Effect of a coalescing aid on the earliest stages of polymer diffusion in poly(butyl acrylate-co-methyl methacrylate) latex films. Polymer 2011, 52, 3984–3993. [Google Scholar] [CrossRef]
- Barbosa, J.V.; Veludo, E.; Moniz, J.; Mendes, A.; Magalhães, F.D.; Bastos, M.M.S.M. Low VOC self-crosslinking waterborne acrylic coatings incorporating fatty acid derivatives. Prog. Org. Coat. 2013, 76, 1691–1696. [Google Scholar] [CrossRef]
- Jiang, S.; Van Dyk, A.; Maurice, A.; Bohling, J.; Fasano, D.; Brownell, S. Design colloidal particle morphology and self-assembly for coating applications. Chem. Soc. Rev. 2017, 46, 3792–3807. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; Poth, U. Coatings Formulation: An. International Textbook; Vincentz Network: Hannover, Germany, 2011; ISBN 9783866308916. [Google Scholar]
- Tsavalas, J.G.; Sundberg, D.C. Hydroplasticization of polymers: Model predictions and application to emulsion polymers. Langmuir 2010, 26, 6960–6966. [Google Scholar] [CrossRef]
- Dron, S.M.; Paulis, M. Tracking hydroplasticization by dsc: Movement of water domains bound to poly(meth)acrylates during latex film formation. Polymers 2020, 12, 2500. [Google Scholar] [CrossRef]
- Feng, J.; Winnik, M.A.; Shivers, R.R.; Clubb, B. Polymer Blend Latex Films: Morphology and Transparency. Macromolecules 1995, 28, 7671–7682. [Google Scholar] [CrossRef]
- Akhmatskaya, E.; Asua, J.M. Dynamic modeling of the morphology of latex particles with in situ formation of graft copolymer. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1383–1393. [Google Scholar] [CrossRef]
- Limousin, E.; Ballard, N.; Asua, J.M. Soft core–hard shell latex particles for mechanically strong VOC-free polymer films. J. Appl. Polym. Sci. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J.-Y. Polymer nanocomposites: The “nano” effect on mechanical properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular polymer chemistry—Scope and perspective. Polym. Int. 2002, 51, 825–839. [Google Scholar] [CrossRef]
- Savyasachi, A.J.; Kotova, O.; Shanmugaraju, S.; Bradberry, S.J.; Ó’Máille, G.M.; Gunnlaugsson, T. Supramolecular Chemistry: A Toolkit for Soft Functional Materials and Organic Particles. Chem 2017, 3, 764–811. [Google Scholar] [CrossRef]
- Lorke, S.; Müller, U.; Meissl, R.; Brüggemann, O. Covalent cross-linking of polymers at room temperature. Int. J. Adhes. Adhes. 2019, 91, 150–159. [Google Scholar] [CrossRef]
- Richard, J.; Maquet, J. Dynamic micromechanical investigations into particle/particle interfaces in latex films. Polymer 1992, 33, 4164–4173. [Google Scholar] [CrossRef]
- Han, L.; Mao, Z.; Wuliyasu, H.; Wu, J.; Gong, X.; Yang, Y.; Gao, C. Modulating the structure and properties of poly(sodium 4-styrenesulfonate)/ poly(diallyldimethylammonium chloride) multilayers with concentrated salt solutions. Langmuir 2012, 28, 193–199. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Retrospective on the future of polyelectrolyte multilayers. Langmuir 2009, 25, 14007–14010. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Kharlampieva, E.; Sukhishvili, S.A. Multilayers of a globular protein and a weak polyacid: Role of polyacid ionization in growth and decomposition in salt solutions. Biomacromolecules 2005, 6, 1782–1788. [Google Scholar] [CrossRef]
- Bossion, A.; Olazabal, I.; Aguirresarobe, R.H.; Marina, S.; Martín, J.; Irusta, L.; Taton, D.; Sardon, H. Synthesis of self-healable waterborne isocyanate-free poly(hydroxyurethane)-based supramolecular networks by ionic interactions. Polym. Chem. 2019, 10, 2723–2733. [Google Scholar] [CrossRef]
- Pinprayoon, O.; Groves, R.; Lovell, P.A.; Tungchaiwattana, S.; Saunders, B.R. Polymer films prepared using ionically crosslinked soft core-shell nanoparticles: A new class of nanostructured ionomers. Soft Matter 2011, 7, 247–257. [Google Scholar] [CrossRef]
- Musa, M.S.; Milani, A.H.; Shaw, P.; Simpson, G.; Lovell, P.A.; Eaves, E.; Hodson, N.; Saunders, B.R. Tuning the modulus of nanostructured ionomer films of core-shell nanoparticles based on poly(: N-butyl acrylate). Soft Matter 2016, 12, 8112–8123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiggelman, I.; Hartmann, P.C. Ionic autocrosslinking of water-based polymer latices: A new concept of acid-base interaction occurring upon film formation. Prog. Org. Coat. 2010, 67, 76–83. [Google Scholar] [CrossRef]
- Turshatov, A.; Adams, J. A new monomeric FRET-acceptor for polymer interdiffusion experiments on polymer dispersions. Polymer 2007, 48, 7444–7448. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J.; Ditchfield, K.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bilgin, S.; Tomovska, R.; Asua, J.M. Effect of ionic monomer concentration on latex and film properties for surfactant-free high solids content polymer dispersions. Eur. Polym. J. 2017, 93, 480–494. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Wang, Y.; Hruska, Z.; Winnik, M.A. Molecular Aspects of Latex Film Formation: An Energy-Transfer Study. Macromolecules 1990, 23, 4082–4087. [Google Scholar] [CrossRef]
- González, E.; Barandiaran, M.J.; Paulis, M. Isolation of the effect of the hairy layer length on the mechanical properties of waterborne coatings. Prog. Org. Coat. 2015, 88, 137–143. [Google Scholar] [CrossRef]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. Kinetics and polymer microstructure of the seeded semibatch emulsion copolymerization of n-butyl acrylate and styrene. Macromolecules 2001, 34, 5147–5157. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zhang, H.; Jiang, G.; Kan, C. Synthesis and characterization of monodispersed P(St-co-DMAEMA) nanoparticles as pH-sensitive drug delivery system. Mater. Sci. Eng. C 2014, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, H.B.; Jethmalani, J.M.; Ford, W.T. Synthesis of Crosslinked Poly(styrene-co-sodium styrenesulfonate) Latexes. J. Polym. Sci. Part. A Polym. Chem. 1994, 32, 1431–1435. [Google Scholar] [CrossRef]
- Dziomkina, N.V.; Hempenius, M.A.; Vancso, G.J. Synthesis of cationic core-shell latex particles. Eur. Polym. J. 2006, 42, 81–91. [Google Scholar] [CrossRef]
- Omer, M.; Khan, M.; Kim, Y.K.; Lee, J.H.; Kang, I.-K.; Park, S.-Y. Biosensor utilizing a liquid crystal/water interface functionalized with poly(4-cyanobiphenyl-4′-oxyundecylacrylate-b-((2-dimethyl amino) ethyl methacrylate)). Colloids Surf. B Biointerfaces 2014, 121, 400–408. [Google Scholar] [CrossRef]
- Salentinig, S.; Jackson, P.; Attalla, M. A computational study of the suppression of ammonia volatility in aqueous systems using ionic additives. Struct. Chem. 2014, 25, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Winnik, M.A.; Farwaha, R.; Rademacher, J. Effect of a Water-Soluble Polymer on Polymer Interdiffusion in P(MMA-co-BA) Latex Films. Macromol. Chem. Phys. 2003, 204, 1933–1940. [Google Scholar] [CrossRef]
- Kobayashi, M.; Rharbi, Y.; Winnik, M.A. Effect of inorganic pigments on polymer interdiffusion in a Low-Tg latex film. Macromolecules 2001, 34, 1855–1863. [Google Scholar] [CrossRef]
- Pinenq, P.; Winnik, M.A.; Ernst, B.; Juhué, D. Polymer diffusion and mechanical properties of films prepared from crosslinked latex particles. J. Coat. Technol. 2000, 72, 45–61. [Google Scholar] [CrossRef]
- González, I.; Asua, J.M.; Leiza, J.R. The role of methyl methacrylate on branching and gel formation in the emulsion copolymerization of BA/MMA. Polymer 2007, 48, 2542–2547. [Google Scholar] [CrossRef]
- Bormashenko, E.; Multanen, V.; Chaniel, G.; Grynyov, R.; Shulzinger, E.; Pogreb, R.; Whyman, G. Phenomenological model of wetting charged dielectric surfaces and its testing with plasma-treated polymer films and inflatable balloons. Colloids Surf. A Physicochem. Eng. Asp. 2015, 487, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.D.; Sperling, L.H.; Klein, A.; Wignall, G.D. Characterization of Film Formation from Direct Mini-emulsified Polystyrene Latex Particles via SANS. Macromolecules 1993, 26, 4624–4631. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Winnik, M.A. Molecular diffusion and latex film formation: An analysis of direct nonradiative energy transfer experiments. J. Chem. Phys. 1991, 95, 2143–2153. [Google Scholar] [CrossRef]
- González, E.; Paulis, M.; Barandiaran, M.J. Effect of controlled length acrylic acid-based electrosteric stabilizers on latex film properties. Eur. Polym. J. 2014, 59, 122–128. [Google Scholar] [CrossRef]










| Surface Charge Density (µC/cm2) | Number of Particles (Np/L) | |||||
|---|---|---|---|---|---|---|
| Latex | Blend C1-1 | Blend C3-3 | Blend C1-3 | Blend P1-1 | Blend P3-3 | Blend P1-3 |
| 1% NaSS | 3 mL | - | 5 mL | 5 mL | - | 5 mL |
| 3% NaSS | - | 3 mL | - | - | 5 mL | - |
| 1% DMAEMA | 5 mL | - | - | 4 mL | - | - |
| 3% DMAEMA | - | 5 mL | 4 mL | - | 3 mL | 4 mL |
| Latex | dp (nm) | Incorporation (% Ionic Monomer) | Surface Charge Density (µC/cm2) | Water-Soluble Species (% Ionic Monomer) |
|---|---|---|---|---|
| 1% NaSS | 275 ± 5 | 70 ± 6 | 16 ± 2 | 30 ± 2 |
| 3% NaSS | 300 ± 4 | 52 ± 3 | 36 ± 4 | 35 ± 3 |
| 1% DMAEMA | 240 ± 5 | 33 ± 4 | 9 ± 2 | 42 ± 10 |
| 3% DMAEMA | 250 ± 2 | 19 ± 4 | 19 ± 3 | 45 ± 10 |
| Latex | Insoluble Polymer (gel) (wt%) | Mw (KDa) | Đ |
|---|---|---|---|
| 1% NaSS | 53 ± 2 | 304 | 2.5 |
| 3% NaSS | 55 ± 1 | 260 | 2.4 |
| 1% DMAEMA | 40 ± 2 | 350 | 2.0 |
| 3% DMAEMA | 30 ± 1 | 340 | 2.1 |
| Blend System | WCA (°) |
|---|---|
| Blend C1-1, reference | 64 ± 4 |
| Blend C1-1, ionic complex | 90 ± 3 |
| Blend C3-3, reference | 64 ± 2 |
| Blend C3-3, ionic complex | 87 ± 3 |
| Blend C1-3, reference | 76 ± 2 |
| Blend C1-3, ionic complex | 90 ± 1 |
| Blend P1-1, reference | 78 ± 1 |
| Blend P1-1, ionic complex | 87 ± 2 |
| Blend P3-3, reference | 86 ± 3 |
| Blend P3-3, ionic complex | 94 ± 4 |
| Blend P1-3, reference | 74 ± 1 |
| Blend P1-3, ionic complex | 91 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argaiz, M.; Ruipérez, F.; Aguirre, M.; Tomovska, R. Ionic Inter-Particle Complexation Effect on the Performance of Waterborne Coatings. Polymers 2021, 13, 3098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183098
Argaiz M, Ruipérez F, Aguirre M, Tomovska R. Ionic Inter-Particle Complexation Effect on the Performance of Waterborne Coatings. Polymers. 2021; 13(18):3098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183098
Chicago/Turabian StyleArgaiz, Maialen, Fernando Ruipérez, Miren Aguirre, and Radmila Tomovska. 2021. "Ionic Inter-Particle Complexation Effect on the Performance of Waterborne Coatings" Polymers 13, no. 18: 3098. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183098