Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
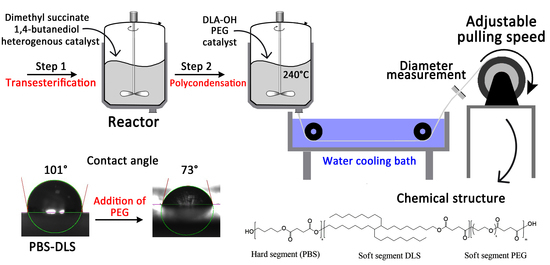
2.2. Synthesis of PBS-DLS-PEG
2.3. Characterization of Chemical Structure
2.4. Gel Permeation Chromatography (GPC)
2.5. Viscosity
2.6. Melt Flow Index (MFI)
2.7. Water Contact Angle
2.8. Thermal Properties
2.9. Dynamic Mechanical Thermal Properties
3. Results and Discussion
3.1. Chemical Structure
3.2. Infrared Spectroscopy (ATR FT-IR)
3.3. Gel Permeation Chromatography (GPC)
3.4. Viscosity and MFI
3.5. Water Contact Angle (WCA)
3.6. Thermal Analysis
3.7. Dynamic Mechanical Properties
3.8. Filament Forming from Polymer Melt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z. Thinking Green: Sustainable Polymers from Renewable Resources. Polymers 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 022001. [Google Scholar] [CrossRef]
- Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; Iraji, A.; Zarei, M.; El Fray, M. Poly(3-hydroxybutyrate)-multiwalled carbon nanotubes electrospun scaffolds modified with curcumin. Polymers 2020, 12, 2588. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prowans, P.; Kowalczyk, R.; Wiszniewska, B.; Czapla, N.; Bargiel, P.; El Fray, M. Bone Healing in the Presence of a Biodegradable PBS-DLA Copolyester and Its Composite Containing Hydroxyapatite. ACS Omega 2019, 4, 19765–19771. [Google Scholar] [CrossRef] [Green Version]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-based polyesters for biomedical applications: A review. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Hariraksapitak, P.; Suwantong, O.; Pavasant, P.; Supaphol, P. Effectual drug-releasing porous scaffolds from 1,6-diisocyanatohexane-extended poly(1,4-butylene succinate) for bone tissue regeneration. Polymer 2008, 49, 2678–2685. [Google Scholar] [CrossRef]
- Ojansivu, M.; Johansson, L.; Vanhatupa, S.; Tamminen, I.; Hannula, M.; Hyttinen, J.; Kellomäki, M.; Miettinen, S. Knitted 3D Scaffolds of Polybutylene Succinate Support Human Mesenchymal Stem Cell Growth and Osteogenesis. Stem Cells Int. 2018, 2018, 5928935. [Google Scholar] [CrossRef] [Green Version]
- Kozlowska, A.; Gromadzki, D.; El Fray, M.; Štěpánek, P. Morphology Evaluation of Biodegradable Copolyesters Based on Dimerized Fatty Acid Studied by DSC, SAXS and WAXS. Fibres Text. East. Eur. 2008, 16, 85–88. [Google Scholar]
- Liverani, L.; Piegat, A.; Niemczyk, A.; El Fray, M.; Boccaccini, A.R. Electrospun fibers of poly(butylene succinate–co–dilinoleic succinate) and its blend with poly(glycerol sebacate) for soft tissue engineering applications. Eur. Polym. J. 2016, 81, 295–306. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- van Dijkhuizen-Radersma, R.; Roosma, J.R.; Kaim, P.; Métairie, S.; Péters, F.L.A.M.A.; De Wijn, J.; Zijlstra, P.G.; De Groot, K.; Bezemer, J.M. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications. J. Biomed. Mater. Res. Part A 2003, 67, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-L.; Jiao, L.; Zhang, J.-J.; Zeng, J.-B.; Yang, K.-K.; Wang, Y.-Z. Poly(butylene succinate)-poly(ethylene glycol) multiblock copolymer: Synthesis, structure, properties and shape memory performance. Polym. Chem. 2012, 3, 800–808. [Google Scholar] [CrossRef]
- Soares, D.Q.P.; Souza, F.G., Jr.; Freitas, R.B.V.; Soares, V.P.; Ferreira, L.P.; Ramon, J.A.; Oliveira, G.E. Praziquantel Release Systems Based on Poly(Butylene Succinate)/Polyethylene Glycol Nanocomposites. Curr. Appl. Polym. Sci. 2017, 1, 45–51. [Google Scholar] [CrossRef]
- Ludueña, L.N.; Fortunati, E.; Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Puglia, D.; Manfredi, L.B.; Pracella, M. Preparation and characterization of polybutylene-succinate/poly(ethylene-glycol)/cellulose nanocrystals ternary composites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Solomon, O.F.; Ciutǎ, I.Z. Determination de la viscosite intrinseque de solutions de polymeres par une simple determination de la viscosite. J. Appl. Polym. Sci. 1962, 6, 683–686. [Google Scholar] [CrossRef]
- Stępień, K.; Miles, C.; McClain, A.; Wiśniewska, E.; Sobolewski, P.; Kohn, J.; Puskas, J.E.; Wagner, H.D.; El Fray, M. Bioopolyesters of Poly(butylene succinate)(PBS) Containing Long-Chain BioBased Glycol Synthesized with Heterogenous Titanium Dioxide Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10623–10632. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Józefczak, A.; Hornowski, T.; Skumiel, A.; Zavisova, V.; Koneracka, M.; Tomasovicova, N.; Timko, M.; Kopcansky, P.; Kelani, H.N. Effect of the Molecular Weight of Poly(ethylene glycol) on the Properties of Biocompatible Magnetic Fluids. Int. J. Thermophys. 2012, 33, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M. Relation of Structure to Thermal and Mechanical Properties. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–73. ISBN 9780323358248. [Google Scholar]
- Jaganathan, S.K.; Mani, M.P.; Prabhakaran, P.; Supriyanto, E.; Ismail, A.F. Production, blood compatibility and cytotoxicity evaluation of a single stage non-woven multicomponent electrospun scaffold mixed with sesame oil, honey and propolis for skin tissue engineering. Int. J. Polym. Anal. Charact. 2019, 24, 457–474. [Google Scholar] [CrossRef]
- Spontak, R.J.; Patel, N.P. Thermoplastic elastomers: Fundamentals and applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]









| Material | Composition | Transesterification | Polycondensation | ||
|---|---|---|---|---|---|
| Time (min) | Temperature (°C) | Time (min) | Temperature (°C) | ||
| PBS-DLS | 70:30 | 80 | 180 | 140 | 236 |
| PBS-DLS-PEG1000 | 70:25:5 | 100 | 216 | 140 | 234 |
| PBS-DLS-PEG1000 | 70:20:10 | 100 | 217 | 140 | 237 |
| PBS-DLS-PEG6000 | 70:25:5 | 100 | 210 | 140 | 236 |
| PBS-DLS-PEG6000 | 70:20:10 | 80 | 217 | 140 | 240 |
| Composition (wt %) | DPh | 1H NMR | GPC | |||||
|---|---|---|---|---|---|---|---|---|
| Copolymer | Theoretical | Calculated | Theoretical | Calculated | Mn (KDa) | Mn (KDa) | Mw (KDa) | Đ |
| PBS-DLS | 70-30 | 64-36 | 8.4 | 6.53 | 53.5 | 49.5 | 127.5 | 2.6 |
| PBS-DLS-PEG1000 | 70-25-05 | 82-08-10 | 10.09 | 8.69 | 20.1 | 43.3 | 128 | 2.8 |
| PBS-DLS-PEG1000 | 70-20-10 | 64-04-32 | 12.61 | 9.52 | 24.8 | 32.2 | 92.4 | 2.9 |
| PBS-DLS-PEG6000 | 70-25-05 | 81-06-13 | 10.09 | 7.30 | 60.2 | 45.6 | 169.7 | 3.7 |
| PBS-DLS-PEG6000 | 70-20-10 | 60-07-33 | 12.61 | 9.43 | 34.1 | 22.7 | 69 | 3.0 |
| Copolymer | Limiting Viscosity Number (dL/g) | MFI (g/10 min) | Time (s) |
|---|---|---|---|
| PBS-DLS (70-30) | 1.015 | 3.16 ± 0.1 | 30 |
| PBS-DLS-PEG1000 (70-25-5) | 0.960 | 13.92 ± 0.8 | 15 |
| PBS-DLS-PEG1000 (70-20-10) | 0.911 | 28.01 ± 1.08 | 15 |
| PBS-DLS-PEG6000 (70-25-5) | 1.008 | 8.66 ± 0.34 | 30 |
| PBS-DLS-PEG6000 (70-20-10) | 0.993 | 136± 1.8 | 15 |
| Copolymer | 15 s | 30 s | 60 s |
|---|---|---|---|
| PBS-DLS (70-30) | 104° ± 3° | 102° ± 4° | 101° ± 4° |
| PBS-DLS-PEG1000 (70-25-5) | 102° ± 2° | 101° ± 2° | 100° ± 2° |
| PBS-DLS-PEG1000 (70-20-10) | 98° ± 2° | 97° ± 3° | 96° ± 3° |
| PBS-DLS-PEG6000 (70-25-5) | 94° ± 3° | 90° ± 3° | 89° ± 2° |
| PBS-DLS-PEG6000 (70-20-10) | 76° ± 4° | 74° ± 3° | 73° ± 3° |
| Samples | Tg (°C) | ΔCp (J/g°C) | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc PBS (%) |
|---|---|---|---|---|---|---|---|
| PBS-DLS 70:30 | −49.9 | 0.197 | 32.0 | 49.48 | 97.0 | 46.31 | 45.4 |
| PBS-DLS-PEG100070:25:5 | −49.6 | 0.335 | 30.3 | 49.64 | 97.9 | 46.70 | 45.8 |
| PBS-DLS-PEG100070:20:10 | −51.6 | 0.297 | 33.1 | 49.92 | 96.9 | 45.37 | 44.5 |
| PBS-DLS-PEG600070:25:5 | −50.6 | 0.344 | 28.7 | 48.93 | 97.7 | 46.08 | 45.2 |
| PBS-DLS-PEG600070:20:10 | −53.8 | 0.274 | 34.9 | 47.52 | 98.6 | 44.01 | 43.1 |
| Copolymers | Max G” (°C) | Max tan δ (°C) |
|---|---|---|
| PBS-DLS 70:30 | −35.8 | −29.8 |
| PBS-DLS-PEG1000 70:25:5 | −36.1 | −31.8 |
| PBS-DLS-PEG1000 70:20:10 | −37.9 | −32.8 |
| PBS-DLS-PEG6000 70:25:5 | −36.9 | −31.7 |
| PBS-DLS-PEG6000 70:20:10 | −38.8 | −33.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, M.; El Fray, M. Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers 2021, 13, 3177. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183177
Zarei M, El Fray M. Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights. Polymers. 2021; 13(18):3177. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183177
Chicago/Turabian StyleZarei, Moein, and Miroslawa El Fray. 2021. "Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights" Polymers 13, no. 18: 3177. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13183177