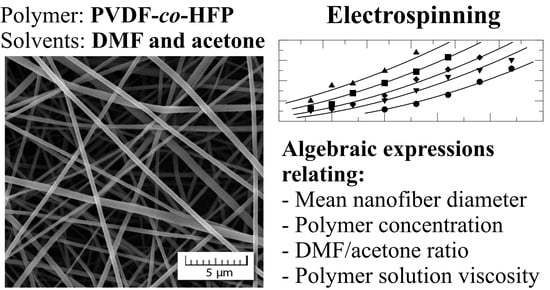
Electrospinning of a Copolymer PVDF-co-HFP Solved in DMF/Acetone: Explicit Relations among Viscosity, Polymer Concentration, DMF/Acetone Ratio and Mean Nanofiber Diameter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Electrospinning Solution
2.3. Process of Electrospinning
2.4. Rheological Measurements
2.5. Characterization of Nanofibrous Mats
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; van d. Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Lalia, B.S.; Guillen-Burrieza, E.; Arafat, H.A.; Hashaikeh, R. Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 428, 104–115. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, N.P.; Chen, J.Y.; Cheng, B.W.; Kang, W.M. The recent research progress and prospect of gel polymer electrolytes in lithium-sulfur batteries. Chem. Eng. J. 2021, 413, 127427. [Google Scholar] [CrossRef]
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Erusappan, E.; Thiripuranthagan, S.; Radhakrishnan, R.; Durai, M.; Kumaravel, S.; Vembuli, T.; Kaleekkal, N.J. Fabrication of mesoporous TiO2/PVDF photocatalytic membranes for efficient photocatalytic degradation of synthetic dyes. J. Environ. Chem. Eng. 2021, 9, 105776. [Google Scholar] [CrossRef]
- Wan, C.; Bowen, C.R. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: The impact of molecular-, micro- and macro-structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the solubility and stability of polyvinylidene fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Yee, W.A.; Kotaki, M.; Liu, Y.; Lu, X. Morphology, polymorphism behavior and molecular orientation of electrospun poly(vinylidene fluoride) fibers. Polymer 2007, 48, 512–521. [Google Scholar] [CrossRef]
- Zheng, J.; He, A.; Li, J.; Han, C.C. Polymorphism control of poly(vinylidene fluoride) through electrospinning. Macromol. Rap. Commun. 2007, 28, 2159–2162. [Google Scholar] [CrossRef]
- María, N.; Maiz, J.; Martínez-Tong, D.; Alegria, A.; Algarni, F.; Zapzas, G.; Hadjichristidis, N.; Müller, A. Phase Transitions in Poly(vinylidene fluoride)/Polymethylene-Based Diblock Copolymers and Blends. Polymers 2021, 13, 2442. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride):Determination, processing and applications. Progr. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Nishiyama, T.; Sumihara, T.; Sasaki, Y.; Sato, E.; Yamato, M.; Horibe, H. Crystalline structure control of poly(vinylidene fluoride) films with the antisolvent addition method. Polym. J. 2016, 48, 1035–1038. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical assisted electrospray/spinning to produce PVP+quercetin microparticles and microfibers. J. Taiwan Inst. Chem. Eng. 2020, 117, 278–286. [Google Scholar] [CrossRef]
- Davis, G.T.; McKinney, J.E.; Broadhurst, M.G.; Roth, S.C. Electric-field-induced phase changes in poly(vinylidene fluoride). J. Appl. Phys. 1978, 49, 4998–5002. [Google Scholar] [CrossRef]
- Lando, J.B.; Olf, H.G.; Peterlin, A. Nuclear magnetic resonance and x-ray determination of the structure of poly(vinylidene fluoride). J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 941–951. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. FTIR studies of beta-phase crystal formation in stretched PVDF films. Polym. Test 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio, R.J.; Lanceros-Méndez, S. Alpha to beta phase transformation and micro-structural changes of PVDF films induced by uniaxial stretch. J. Macromol. Sci. Part B Physics 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.G.; Lanceros-Méndez, S. Influence of Processing Conditions on Polymorphism and Nanofiber Morphology of Electroactive Poly(vinylidene fluoride) Electrospun Membranes. Soft Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Azimi, S.; Fashandi, H. Enhanced ferroelectric properties of electrospun poly(vinylidene fluoride) nanofibers by adjusting processing parameters. RSC Adv. 2015, 5, 61277–61283. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. Solubility parameters of poly(vinylidene fluoride). J. Polym. Sci. Part B: Polym. Phys. 1988, 26, 785–794. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gee, S.; Johnson, B.; Smith, A. Optimizing electrospinning parameters for piezoelectric PVDF nanofiber membranes. J. Membr. Sci. 2018, 563, 804–812. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Li, J.Q.; Yuan, X.Y.; Li, X.; Zhang, Y.Y.; Sheng, J. Preparation and properties of electrospun poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2005, 97, 466–474. [Google Scholar] [CrossRef]
- Baqeri, M.; Abolhasani, M.M.; Mozdianfard, M.R.; Guo, Q.P.; Oroumei, A.; Naebe, M. Influence of processing conditions on polymorphic behavior, crystallinity, and morphology of electrospun poly(vinylidene fluoride) nanofibers. J. Appl. Polym. Sci. 2015, 132, 42304. [Google Scholar] [CrossRef]
- Chen, H.-C.; Tsai, C.-H.; Yang, M.-C. Mechanical properties and biocompatibility of electrospun polylactide/poly(vinylidene fluoride) mats. J. Polym. Res. 2011, 18, 319–327. [Google Scholar] [CrossRef]
- Al Halabi, F.; Gryshkov, O.; Kuhn, I.A.; Kapralova, V.; Glasmacher, B. Force induced piezoelectric effect of polyvinylidene fluoride and polyvinylidene fluoride-co-trifluoroethylene nanofibrous scaffolds. Int. J. Artif. Organs 2018, 41, 811–822. [Google Scholar] [CrossRef]
- Orkwis, J.A.; Wolf, A.K.; Shahid, S.M.; Smith, C.; Esfandiari, L.; Harris, G.M. Development of a Piezoelectric PVDF-TrFE Fibrous Scaffold to Guide Cell Adhesion, Proliferation, and Alignment. Macromol. Biosci. 2020, 20, 2000197. [Google Scholar] [CrossRef]
- He, F.; Fan, J.; Chan, L.H. Preparation and characterization of electrospun poly(vinylidene fluoride)/poly(methyl methacrylate) membrane. High Perform. Polym. 2014, 26, 817–825. [Google Scholar] [CrossRef]
- Nuamcharoen, P.; Kobayashi, T.; Potiyaraj, P. Influence of volatile solvents and mixing ratios of binary solvent systems on morphology and performance of electrospun poly(vinylidene fluoride) nanofibers. Polym. Int. 2021, 70, 1465–1477. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of uniform polystyrene fibers: The effect of solvent conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, L.; Gao, Q. Preparation of PVDF nanofibrous membrane and its waterproof and breathable property. Adv. Mater. Res. 2013, 796, 327–330. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Choi, S.; Kim, H.S. Structural deformation of PVDF nanoweb due to electrospinning behavior affected by solvent ratio. e-Polymers 2018, 18, 339–345. [Google Scholar] [CrossRef]
- Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, Y.R. An electrospun poly(vinylidene fluoride) nanofibrous membrane and its battery applications. Adv. Mater. 2003, 15, 2027–2032. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Brinke, G.T.; Loos, K. Well-defined copolymers based on poly(vinylidene fluoride): From preparation and phase separation to application. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2861–2877. [Google Scholar] [CrossRef]
- Dognani, G.; Cabrera, F.C.; Job, A.E.; Agostini, D.L.D.S. Morphology of Electrospun Non-Woven Membranes of Poly(vinylidene fluoride-co-hexafluoropropylene): Porous and Fibers. Fibers Polym. 2019, 20, 512–519. [Google Scholar] [CrossRef]
- Noor, M.M.; Buraidah, M.H.; Careem, M.A.; Majid, S.R.; Arof, A.K.F. An optimized poly(vinylidene fluoride-hexafluoropropylene)–NaI gel polymer electrolyte and its application in natural dye sensitized solar cells. Electrochim. Acta 2014, 121, 159–167. [Google Scholar] [CrossRef]
- Neese, B.; Wang, Y.; Chu, B.; Ren, K.; Liu, S.; Zhang, Q.M.; Huang, C.; West, J. Piezoelectric responses in poly(vinylidene fluoride/hexafluoropropylene) copolymers. Appl. Phys. Lett. 2007, 90, 242917. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- García-Fernández, L.; García-Payo, M.C.; Khayet, M. Mechanism of formation of hollow fiber membranes for membrane distillation: 1. Inner coagulation power effect on morphological characteristics. J. Membr. Sci. 2017, 542, 456–468. [Google Scholar] [CrossRef]
- Kim, I.; Kim, B.S.; Nam, S.; Lee, H.-J.; Chung, H.K.; Cho, S.M.; Luu, T.H.T.; Hyun, S.; Kang, C. Cross-linked poly(vinylidene fluoride-cohexafluoropropene) (PVDF-co-HFP) gel polymer electrolyte for flexible Li-ion battery integrated with organic light emitting diode (OLED). Materials 2018, 11, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Alcoutlabi, M.; Watson, J.V.; Zhang, X. Electrospun nanofiber-coated separator membranes for Lithium-ion rechargeable batteries. J. Appl. Polym. Sci. 2013, 129, 1939–1951. [Google Scholar] [CrossRef]
- Singh, R.; Janakiraman, S.; Agrawal, A.; Ghosh, S.; Venimadhav, A.; Biswas, K. An amorphous poly(vinylidene fluoride-co-hexafluoropropylene) based gel polymer electrolyte for magnesium ion battery. J. Electroanal. Chem. 2020, 858, 113788. [Google Scholar] [CrossRef]
- Lee, D.; Woo, Y.C.; Park, K.H.; Phuntsho, S.; Tijing, L.D.; Yao, M.; Shim, W.-G.; Shon, H.K. Polyvinylidene fluoride phase design by two-dimensional boron nitride enables enhanced performance and stability for seawater desalination. J. Membr. Sci. 2019, 598, 117669. [Google Scholar] [CrossRef]
- Su, C.-I.; Shih, J.-H.; Huang, M.-S.; Wang, C.-M.; Shih, W.-C.; Liu, Y.-S. A study of hydrophobic electrospun membrane applied in seawater desalination by membrane distillation. Fibers Polym. 2012, 13, 698–702. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Johir, M.A.H.; Choi, J.-S.; Shon, H.K. A novel dual-layer bicomponent electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Chem. Eng. J. 2014, 256, 155–159. [Google Scholar] [CrossRef]
- Tas, M.; Memon, H.; Xu, F.; Ahmed, I.; Hou, X. Electrospun nanofibre membrane based transparent slippery liquid-infused porous surfaces with icephobic properties. Coll. Surf. A 2020, 585, 124177. [Google Scholar] [CrossRef]
- Shahabadi, S.M.S.; Rabiee, H.; Seyedi, S.M.; Mokhtare, A.; Brant, J.A. Superhydrophobic dual layer functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PH) nanofibrous membrane for high flux membrane distillation. J. Membr. Sci. 2017, 537, 140–150. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, C.; Lu, X.; Ng, D.; Truong, Y.B.; Xie, Z. Activated carbon enhanced hydrophobic/hydrophilic dual-layer nanofiber composite membranes for high-performance direct contact membrane distillation. Desalination 2018, 446, 59–69. [Google Scholar] [CrossRef]
- CAMPUS Datasheet Kynar Flex® 2801-00. Available online: https://www.campusplastics.com/campus/en/datasheet/Kynar+Flex%C2%AE+2801-00/ARKEMA/179/5d7bdb8c (accessed on 4 October 2021).
- Peer, P.; Stenicka, M.; Pavlinek, V.; Filip, P. The storage stability of polyvinylbutyral solutions from an electrospinnability standpoint. Polym. Degr. Stab. 2014, 105, 134–139. [Google Scholar] [CrossRef]
- Meringolo, C.; Poerio, T.; Fontananova, E.; Mastropietro, T.F.; Nicoletta, F.P.; de Filpo, G.; Curcio, E.; di Profio, G. Exploiting fluoropolymers immiscibility to tune surface properties and mass transfer in blend membranes for membrane contactor applications. ACS Appl. Polym. Mater. 2019, 1, 326–334. [Google Scholar] [CrossRef]









| Component | δd [MPa1/2] | δp [MPa1/2] | δh [MPa1/2] |
|---|---|---|---|
| PVDF | 17.2 | 12.5 | 9.2 |
| PVDF-co-HFP | 19.9 | 12.8 | 11.6 |
| DMF | 17.4 | 13.7 | 11.3 |
| acetone | 15.5 | 10.4 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, P.; Zelenkova, J.; Peer, P. Electrospinning of a Copolymer PVDF-co-HFP Solved in DMF/Acetone: Explicit Relations among Viscosity, Polymer Concentration, DMF/Acetone Ratio and Mean Nanofiber Diameter. Polymers 2021, 13, 3418. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193418
Filip P, Zelenkova J, Peer P. Electrospinning of a Copolymer PVDF-co-HFP Solved in DMF/Acetone: Explicit Relations among Viscosity, Polymer Concentration, DMF/Acetone Ratio and Mean Nanofiber Diameter. Polymers. 2021; 13(19):3418. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193418
Chicago/Turabian StyleFilip, Petr, Jana Zelenkova, and Petra Peer. 2021. "Electrospinning of a Copolymer PVDF-co-HFP Solved in DMF/Acetone: Explicit Relations among Viscosity, Polymer Concentration, DMF/Acetone Ratio and Mean Nanofiber Diameter" Polymers 13, no. 19: 3418. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193418