Crazing Effect on the Bio-Based Conducting Polymer Film
Abstract
:1. Introduction
2. Characterization Techniques
2.1. Materials
2.2. Preparation of Crazed PLA/PAni Film and Non-Crazed PLA/PAni Film
2.3. Characterization Techniques
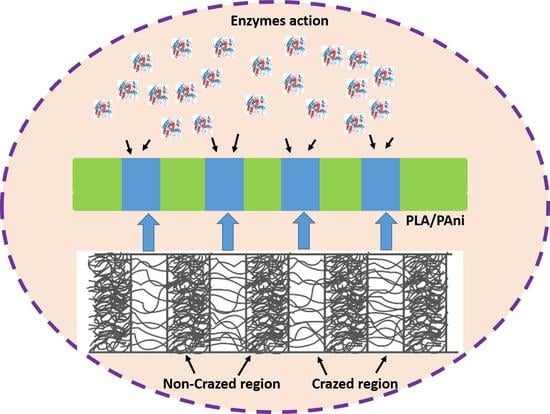
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolman, B.; Boon, A.R.; Briere, C.; Group, E.; Prins, T. Oceans Report Addressing SDG14 Issues with Factual Data and State of the Art Knowledge; Spring: Deltares, The Netherlands, 2018; pp. 1–93. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordono, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggalí, J.; Engel, E.; Del Valle, L.J.; Alemán, C. Electrospun Conducting and Biocompatible Uniaxial and Core-Shell Fibers Having Poly(Lactic Acid), Poly(Ethylene Glycol), and Polyaniline for Cardiac Tissue Engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability Standards for Carrier Bags and Plastic Films in Aquatic Environments: A Critical Review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef] [Green Version]
- Rocca-Smith, J.R.; Whyte, O.; Brachais, C.H.; Champion, D.; Piasente, F.; Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Karbowiak, T. Beyond Biodegradability of Poly(Lactic Acid): Physical and Chemical Stability in Humid Environments. ACS Sustain. Chem. Eng. 2017, 5, 2751–2762. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. The Contribution of Eco-Friendly Bio-Based Blends on Enhancing the Thermal Stability and Biodegradability of Poly(Lactic Acid). J. Clean. Prod. 2018, 198, 987–995. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline Nanofibers: Broadening Applications for Conducting Polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.; Phang, S.W.; Baharum, A. Effects of Synthesised Polyaniline (PAni) Contents on the Antistatic Properties of PAni-Based Polylactic Acid (PLA) Films. RSC Adv. 2020, 10, 39693–39699. [Google Scholar] [CrossRef]
- Hodges, H.W.M. The Formation of Crazing in Some Early Chinese Glazed Wares. Stud. Conserv. 1988, 33, 155–157. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Bagrov, D.V.; Bakirov, A.V.; Yarysheva, L.M.; Chvalun, S.N.; Volynskii, A.L. Effect of Initial Polypropylene Structure on Its Deformation via Crazing Mechanism in a Liquid Medium. Eur. Polym. J. 2018, 100, 233–240. [Google Scholar] [CrossRef]
- Diani, J.; Gall, K. Finite Strain 3D Thermoviscoelastic Constitutive Model. Society 2006, 46, 1–10. [Google Scholar]
- Barton-Pudlik, J.; Czaja, K.; Grzymek, M.; Lipok, J. Evaluation of Wood-Polyethylene Composites Biodegradability Caused by Filamentous Fungi. Int. Biodeterior. Biodegrad. 2017, 118, 10–18. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Takahashi, S.; Takeno, A. Growth of Craze Phase and Control of Void Diameter by Laplace-Pressure in Crazing Films. Jpn. J. Appl. Phys. 2019, 58, SAAD05. [Google Scholar] [CrossRef]
- Rohani, R.; Izni Yusoff, I.; Adlyna Mey Efdi, F.; Mohd Junaidi, M.U. Polyaniline Composite Membranes Synthesis in Presence of Various Acid Dopants for Pressure Filtration Preparation of Polyaniline Composite Membranes Polyaniline Membranes Were Synthesized onto Microporous PVDF Supports in Presence of Various Acid Dopants. UKM Eng. J. 2017, 29, 1–12. [Google Scholar]
- Wan Ishak, W.H.; Rosli, N.A.; Ahmad, I. Influence of Amorphous Cellulose on Mechanical, Thermal, and Hydrolytic Degradation of Poly(Lactic Acid) Biocomposites. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Shahdan, D.; Ahmad, S.; Chen, R.S.; Omar, A.; Zailan, F.D.; Abu Hassan, N.A. Mechanical Performance, Heat Transfer and Conduction of Ultrasonication Treated Polyaniline Bio-Based Blends. Int. Commun. Heat Mass Transf. 2020, 117, 104742. [Google Scholar] [CrossRef]
- Zhang, H. Characterization of Electrospun Poly(Lactic Acid)/Polyaniline Blend Nanofibers. Adv. Mater. Res. 2011, 332–334, 317–320. [Google Scholar] [CrossRef]
- Luo, W.; Liu, W. Incubation Time to Crazing in Stressed Poly(Methyl Methacrylate). Polym. Test. 2007, 26, 413–418. [Google Scholar] [CrossRef]
- Hart, K.R.; Dunn, R.M.; Sietins, J.M.; Hofmeister Mock, C.M.; Mackay, M.E.; Wetzel, E.D. Increased Fracture Toughness of Additively Manufactured Amorphous Thermoplastics via Thermal Annealing. Polymer 2018, 144, 192–204. [Google Scholar] [CrossRef]
- Deblieck, R.A.C.; Van Beek, D.J.M.; Remerie, K.; Ward, I.M. Failure Mechanisms in Polyolefines: The Role of Crazing, Shear Yielding and the Entanglement Network. Polymer 2011, 52, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Luo, W.; Yin, B. Strain-Amplitude and Strain-Rate Dependent Craze Damage of Poly(Methyl Methacrylate). Polym. Polym. Compos. 2014, 22, 737–742. [Google Scholar]
- Lim, H.; Hoag, S.W. Plasticizer Effects on Physical-Mechanical Properties of Solvent Cast Soluplus® Films. AAPS PharmSciTech 2013, 14, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Yarysheva, A.Y.; Arzhakova, O.V.; Yarysheva, L.M.; Volynskii, A.L. Biaxial Tensile Drawing of Poly(Ethylene Terephthalate) via Environmental Crazing as a Method for Creating a Porous Structure. Polymer 2018, 158, 243–253. [Google Scholar] [CrossRef]
- Arzhakova, O.V.; Dolgova, A.A.; Yarysheva, L.M.; Volynskii, A.L.; Bakeev, N.F. Development of a Stable Open-Porous Structure in the Solvent-Crazed High-Density Polyethylene. Inorg. Mater. Appl. Res. 2011, 2, 493–498. [Google Scholar] [CrossRef]
- Kurbanov, M.A.; Gol’dade, V.A.; Zotov, S.V.; Ramazanova, I.S.; Nuraliev, A.F.; Yakhyaev, F.F.; Yusifova, U.V.; Khudayarov, B.G. Generation of Craze-Formation Centers in Polymer Films under the Action of Electric Discharge Plasma. Tech. Phys. 2018, 63, 965–969. [Google Scholar] [CrossRef]
- Newman, S.B.; Wolock, I. Optical Studies of Crazed Plastic Surfaces. J. Res. Natl. Bur. Stand. 1957, 58, 339. [Google Scholar] [CrossRef]
- Kausch, H.H.; Gensler, R.; Grein, C.; Plummer, C.J.G.; Scaramuzzino, P. Crazing in Semicrystalline Thermoplastics. J. Macromol. Sci. Phys. 1999, 38, 37–41. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.S. Investigation of Damage Mechanisms of Polyethylene Film during Stable Crack Growth. Mater. Res. Express 2019, 6, 125343. [Google Scholar] [CrossRef]
- Donth, E.; Michler, G.H. Discussion of Craze Formation and Growth in Amorphous Polymers in Terms of the Multiplicity of Glass Transition at Low Temperatures. Colloid Polym. Sci. 1989, 267, 557–567. [Google Scholar] [CrossRef]
- Stoffel, F.; Weschenfelder, E.F.; Camassola, M.; Piemolini-Barreto, L.T.; Zeni, M. Influence of Plasticizers in Enzymatic Degradation and Water Resistance of Starch Foam Trays Obtained by Thermal Expansion. J. Polym. Environ. 2019, 27, 739–746. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta Arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of Glycerol and Corn Oil on Physicochemical Properties of Polysaccharide Films—A Comparative Study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Klein, F.; Sommerfeld, A. Technical Applications. In The Theory of the Top. Volume IV; Birkhäuser: Basel, Switzerland, 2014; pp. 761–936. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. EUROPEAN POLYMER New Emerging Trends in Synthetic Biodegradable Polymers—Polylactide: A Critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Bagrov, D.V.; Yarysheva, A.Y.; Rukhlya, E.G.; Yarysheva, L.M.; Volynskii, A.L.; Bakeev, N.F. Atomic Force Microscopic Study of the Structure of High-Density Polyethylene Deformed in Liquid Medium by Crazing Mechanism. J. Microsc. 2014, 253, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosli, N.A.; Ahmad, I.; Anuar, F.H.; Abdullah, I. Effectiveness of Cellulosic Agave Angustifolia Fibres on the Performance of Compatibilised Poly(Lactic Acid)-Natural Rubber Blends. Cellulose 2019, 26, 3205–3218. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegyesi, N.; Zhang, Y.; Kohári, A.; Polyák, P.; Sui, X.; Pukánszky, B. Enzymatic Degradation of PLA/Cellulose Nanocrystal Composites. Ind. Crops Prod. 2019, 141, 111799. [Google Scholar] [CrossRef] [Green Version]
- Pathak, V.M.; Navneet. Review on the Current Status of Polymer Degradation: A Microbial Approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Huang, Q.; Hiyama, M.; Kabe, T.; Kimura, S.; Iwata, T. Enzymatic Self-Biodegradation of Poly(l-Lactic Acid) Films by Embedded Heat-Treated and Immobilized Proteinase K. Biomacromolecules 2020, 21, 3301–3307. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Kulkarni, A.; Reiche, J.; Hartmann, J.; Kratz, K.; Lendlein, A. Selective Enzymatic Degradation of Poly(ε-Caprolactone) Containing Multiblock Copolymers. Eur. J. Pharm. Biopharm. 2008, 68, 46–56. [Google Scholar] [CrossRef]
- Kim, K.; Elsawy, M.A.; Kim, K.; Park, J.; Deep, A. Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites Hydrolytic Degradation of Polylactic Acid (PLA) and Its Composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar]
- Benali, S.; Aouadi, S.; Dechief, A.L.; Murariu, M.; Dubois, P. Key Factors for Tuning Hydrolytic Degradation of Polylactide/Zinc Oxide Nanocomposites. Nanocomposites 2015, 1, 51–61. [Google Scholar] [CrossRef]









| Sample | Content of Sampling Tube |
|---|---|
| Non-crazed PLA/PAni | 0.5 mg of Proteinase K and 1.0 g of sodium azide in 10 mL Tris-HCl (pH 8.0) |
| Control sample | 1.0 g Sodium azide in 10 mL Tris-HCl (pH 8.0) |
| Crazed PLA/PAni | 0.5 mg Proteinase K and 1.0 g sodium azide in 10 mL Tris-HCl (pH 8.0) |
| Functional Group | Wavenumber (cm−1) | ||
|---|---|---|---|
| PLA | PAni | PLA/PAni | |
| N−H stretching | - | 3215 | - |
| C=O stretching | 1746 | - | 1574–1576 |
| C=C stretching of quinoid and benzenoid | - | 1584, 1487 | 1457, 1354 |
| C−N stretching | - | 1244 | 1260–1301 |
| C−H stretching | 867 | 810 | 872 |
| Properties | Non-Crazed PLA/PAni | Crazed PLA/PAni | Remarks |
|---|---|---|---|
| Optical microscope | - | Lamellae presents | Confirmed crazes presented |
| SEM | ↓ porosity and smooth | ↑ porosity & diagonal crack | Confirmed crazes presented |
| Tensile strength | 20.02 MPa (similar) | 19.25 MPa (similar) | After crazed, high tensile strength remained |
| Young’s modulus | 1113 MPa | 651 MPa | After crazed, modulus ↓, stiffness decreased |
| Tensile Strain | 100% | 250% | After crazed, 150% increment, better flexibility |
| Biodegradation | 31% | 42% | After crazed, % biodegradation ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, P.-Y.; Takeno, A.; Takahashi, S.; Phang, S.-W.; Baharum, A. Crazing Effect on the Bio-Based Conducting Polymer Film. Polymers 2021, 13, 3425. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193425
Wong P-Y, Takeno A, Takahashi S, Phang S-W, Baharum A. Crazing Effect on the Bio-Based Conducting Polymer Film. Polymers. 2021; 13(19):3425. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193425
Chicago/Turabian StyleWong, Pei-Yi, Akiyoshi Takeno, Shinya Takahashi, Sook-Wai Phang, and Azizah Baharum. 2021. "Crazing Effect on the Bio-Based Conducting Polymer Film" Polymers 13, no. 19: 3425. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13193425