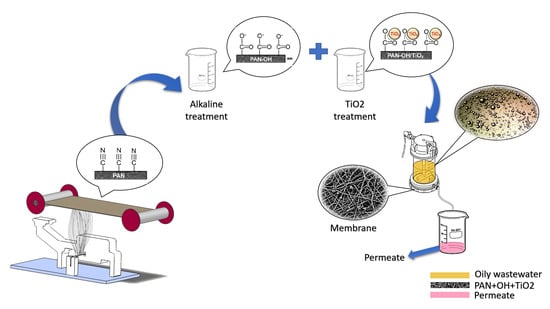
3.1. Membrane Characterisation
SEM images obtained for the unmodified control membranes (S1 and S2;
Figure 7) and modified membranes (S3–S6;
Figure 8) showed no significant difference in fibre morphology under different nanofibre web densities (see
Table 1), suggesting that collector speed has no significant effect on fibre diameter. After modification in alkaline solution, however, fibre diameter increased slightly, and increased still further following TiO
2 treatment (
Table 1). In our modification system, the nitrile groups (–C≡N) were hydrolysed and converted into carboxyl groups (–COO–) following immersion in the alkaline solutions, which improved the surface’s hydrophilic and charging properties. In addition, hydrolysis of PAN resulted in membrane swelling, reducing the pore size and making the membrane surface smoother [
4,
22]. Functional carboxyl groups attract TiO
2, causing it to graft onto the membrane surface (
Figure 8). This configuration of carboxylic groups with TiO
2 is known as bidentate chelation, bidentate bridging, and C=O or C–OH monodentate complexation [
23,
24].
SEM images indicated that TiO
2 particles were not well distributed on the treated membranes, with clear aggregation on the fibre’s surface (
Figure 8; S4 and S6), suggesting that PAN hydrolysis following alkaline treatment was irregular. In a previous study [
18], a more regular distribution of TiO
2 nanoparticles was observed on the surface of polyvinylidene fluoride (PVDF) nanofibres following alkaline treatment as dehydrofluorination of the PVDF surface was more regular, allowing improved attachment of TiO
2 nanoparticles to hydrophilic –OH groups on the fibres.
The peaks at 2242 and 1452 cm
–1 in the FT-IR polyacrylonitrile membrane spectra before and after modification were due to bending of the –CN group and CH
2, respectively (
Figure 9). The peaks decreased slightly in both the modified S3 and S5 membranes, confirming partial (incomplete) hydrolysis and conversion of CN to COOH groups. On the other hand, these same peaks were reduced sharply in all cases following alkaline and TiO
2 treatment. Following reaction with the alkaline solution, hydrolysis resulted in peaks between 1600 and 1700 cm
−1, which probably indicates the presence of –COONa and -CONH
2 groups in the S3 and S5 modified membranes. It has previously been shown that the –CN group on PAN nanofibres can be transformed into amide (–CONH
2) and carboxylic sodium (–COONa) groups following being hydrolysis in alkaline [
25].
The peak at 1735 cm−1 indicates C=O stretching. This same peak was also observed in pristine PAN membranes and is attributable to vinyl acetate monomers. The peak increased slightly following alkaline treatment due to the formation of carboxyl groups.
All peaks characteristic of PAN (2242, 1735, and 1452 cm−1) in the S4 and S6 samples were significantly reduced following treatment due to the attachment of TiO2 nanoparticles to the alkaline-treated membrane surface.
Membrane selectivity, permeability, fouling and rejection are primarily dependant on membrane pore size and operating conditions (pH, temperature, etc.). In this study, all operating conditions were kept the same and all modified samples were preserved in distilled water; thus, any differences are likely to be due to pore size alone. Our results indicated that average membrane pore size decreased as nanofibre web density increased (
Table 1), with an initial pore size for control membrane S1 (1 g/m
2) of 1.74 ± 0.1 µm decreasing to 0.9 ± 0.08 µm in control membrane S2 (3 g/m
2) due to its higher fibre density and more compact structure. The final pore size following modification (S3–S6) was reduced even further (by around 50%;
Table 2), due to fibre swelling following alkaline treatment and TiO
2 coating the fibres.
As most separation units work under high pressures, a standard membrane must be able to resist damage or bursting under external pressure; hence, we tested bursting pressure to determine the degree of delamination for each layer and to assess lamination process quality. Both S1 and S2 control membranes showed high bursting strength (
Table 3), with at least two bars (applied from the rear side) needed to separate the nanofibre layer from the support. In such cases, modification reduced adhesion between the layers, thereby reducing its tensile properties. Based on these results, we consider 90 kPa to be the maximum recommended pressure for backwash cleaning of membranes prepared in this way.
As observed in previous studies [
18,
26], a higher web density generally results in increased fibre compaction, which reduces air permeability. As such, we undertook an air permeability test following the lamination process in order to assess to what degree the melted adhesive web clogged the nanofibre pores. As expected, the increased density of sample S2 reduced its permeability (
Table 3); however, S2 displayed a better bursting strength than S1, giving it greater mechanical strength and better handling properties, hence S2 was chosen for further surface modification. The air permeability of all samples was reduced following modification, presumably due to the reduced pore size caused by fibre swelling after alkaline treatment and adhesion of nanoparticles on the fibre.
To assess the wettability of the membranes, the water contact angle of both modified and unmodified membranes was measured before and after separation took place. Our results showed that the unmodified membranes displayed a lower contact angle after separation (
Table 4), probably due to surface contamination and the effects of the non-ionic Triton X-100 surfactant on the membrane. All the modified membranes (S3–S6) displayed surface hydrophilicity (
Table 4) due to the formation of the strongly hydrophilic carboxyl group (COOH) on the surface of the membranes. While alkaline treatment alone (S3 and S5) was enough to promote super hydrophilic functionality, previous studies have shown that TiO
2 also exhibits super hydrophilicity when illuminated under UV light (e.g., [
27]); hence, the use of an external source of UV light might be expected to further enhance the super hydrophilic properties of membranes S4 and S6 (TiO
2 treated). However, in a previous study [
28] in which TiO
2 nanoparticles were grafted onto a membrane surface following plasma and alkaline treatment, there was no apparent impact on membrane separation performance following TiO
2 treatment with and without UV-light, possibly due to the particle size used, which can affect its photocatalytic activity. In this work, the membranes were prepared in the laboratory and filtration was done under natural sunlight; hence, we were unable to assess the impact of external UV light treatment on the TiO
2 treated membranes. It should also be noted that membrane contact angle is a function not only of the nanofibre itself but also the components used to construct it. In our samples, it is possible that the adhesive web used in the lamination process may have affected the membrane contact angle, and hence hydrophilicity, by covering the surface of the nanofibres.
3.2. Separation Test
One disadvantage of units such as that used in our test is that the membrane in the dead-end filtration unit gradually becomes fouled as contaminants collect on the membrane surface, thereby reducing its permeability. To assess the self-cleaning and selectivity capabilities of each membrane, we repeated the separation process on each membrane three times without changing the membrane between cycles, with each cycle consisting of a wash through with distilled water followed by a feed wash with the oil/water emulsion.
As might be expected, a comparison of the two control membranes indicated that membrane S2 (3 g/m
2) was less permeable that S1 (1 g/m2) owing to its more compact nanofibre web and reduced pore size, with initial readings ca. one third of those for S1 (
Figure 10). Similar results were obtained in a previous study using membranes prepared with polyamide 6 (PA6) nanofibres [
29], whereby a 1.11 g/m
2 PA6 membrane displayed permeability almost twice that of a 2.31 g/m
2 PA6 membrane. While S1 permeability remained virtually unchanged over successive cycles, with just a slight reduction probably caused by fouling, S2 showed an increase in permeability of ca. two-fold over the second and third cycles, most likely due to the surfactant. Nevertheless, S2 permeability remained at around two thirds that of S1. Aside from the first run for S2, permeability readings remained roughly similar over successive cycles (
Figure 10), most likely due to the non-ionic Triton X-100 surfactant added at the suspension preparation stage (see also results for ‘wettability’ above). In a previous study, Fane et al. [
30] found that the addition of a non-ionic surfactant increased membrane flux by 20% and also made membrane cleaning easier. On the other hand, other studies have reported that surfactant addition can decrease membrane flux due to concentration polarisation and trapping of adsorbate surfactant molecules in the membrane pores [
31,
32,
33,
34]. It is possible that these conflicting results for flux and permeability arise from the actual amount of non-ionic surfactant used in the emulsion.
While treatment with alkaline improved permeability in both test membranes, there was a clear difference depending on the alkaline used, with permeability of those treated with NaOH (S3) improving just two-fold while those treated with KOH (S5) improving ca. eight-fold (
Figure 11). This difference in the degree of hydrolysis, which is due to differences in the attack strength of the hydroxyl group, was also confirmed in the study of Zhang et al. [
35], who recorded clear differences in hydrolysis levels between membranes treated with KOH, NaOH and lithium hydroxide (LiOH), with the degree of hydrolysis mainly in line with the order of basicity intensity.
In addition to increasing membrane hydrophilicity, alkaline treatment has previously been shown to improve membrane chemical resistance to common organic solvents, including DMF (
N,
N-Dimethylformamide), DMSO (dimethyl sulfoxide) and NMP (
N-Methyl-2-pyrrolidone) [
3]. Moreover, by decreasing the diameter of PAN fibres to the nano-scale, fibre specific surface area is increased, increasing the amount of carboxyl (–COOH) groups available on the surface [
25].
In our own test, the KOH treated membrane (S3) not only showed better flux and permeability than the NaOH membrane (S5), its membrane permeability increased over successive cycles, while that of the NaOH membrane decreased (
Figure 11). Roughly similar results were recorded for the NaOH membrane following TiO
2 nanoparticle grafting (S4), with permeability remaining similar (ca. two-fold increase) and decreasing over successive cycles (
Figure 11). The TiO
2-grafted KOH membrane (S6), however, showed a further increase in permeability, with a ca. thirteen-fold increase over the non-treated control membrane (S2) and a ca. one-and-a-half-fold increase over the membrane treated with KOH alone (S5;
Figure 11). As such, a positive effect from grafting TiO
2 nanoparticles to alkaline treated membranes was only observed in the case of KOH treatment, most likely as the greater increase in hydrolysis resulting from KOH treatment allowed more TiO
2 nanoparticles to attach to the membrane surface.
As shown previously by Yalcinkaya et al. [
11], laminated PAN membranes are hydrophilic, i.e., they repel oil when filtering oil/water mixtures; further, as our own results show, the surfaces of alkaline-treated membranes also show enhanced hydrophilic/oleophobic properties when filtering oil/water emulsions (
Table 5). Surprisingly, however, further enhancement with TiO
2 reduced membrane selectivity, with oil droplets being found in the permeate from both KOH and NaOH membranes after separation (
Table 5). There are a number of possible reasons for this apparent increase in oleophilicity. Firstly, TiO
2 is likely to have increased surface roughness. Shuai et al. [
36], for example, found that addition of TiO
2 nanoparticles to the surface of a polyurethane sponge increased surface roughness and hydrophobicity, while Gao et al. [
37] noted that increased surface roughness caused by grafting TiO
2 particles to the surface of cotton increased the material’s oleophilic properties. As stated earlier, UV illumination of TiO
2 particles has the potential to significantly increase surface hydrophilicity and oleophilicity (e.g., see [
27]), making them highly amphiphilic. Wang et al. [
38] noted that changes in wettability occurred on both anatase and rutile TiO
2 surfaces in the form of either polycrystals or single crystals, independent of TiO
2 particle photocatalytic activity, and that this change in wettability resulted in zero contact angles for both water and oily liquids [
39]. The high amphiphilicity of this TiO
2 surface material was maintained, even after storage in a dark place for a few days. In our experiment, the filtration experiments took place under sunlight and, as such, natural light UV may have increased the amphiphilicity of our modified membranes; however, the low levels from sunlight alone do not appear to have been sufficient to maintain the amphiphilic surface.
Our results indicate that alkaline treatment improves PAN membrane hydrophilicity and flux and deceases overall pore size, while additional grafting of TiO2 nanoparticles decreases membrane selectivity but improves permeability and fouling resistance. We conclude that separation between the hydrophilic and oleophilic phases accounts for the amphiphilic character of the TiO2 grafted membrane surfaces used here, with the hydrophilic part improving membrane flux and permeability and potentially reducing the cake layer that forms on the membrane surface. It is likely that the hydrophobic side becomes attached to the hydrophobic membrane matrix, and that the hydrophilic side then becomes stabilised on the surface, resulting in reduced interaction between the foulant and membrane surface. As such, surface modification of the membrane helped improve the membrane’s anti-fouling properties while maintaining its high flux and rejection properties, with the result that the membrane showed high permeability and self-cleaning properties, even when faced with high concentrations of high-viscosity oil in emulsion.
Numerous studies have shown that membrane separation performance against oil-water emulsions varies greatly depending on the amount of oil in emulsion, the type of oil (low viscosity, high viscosity), droplet size, amount of surfactant, type of surfactant and pressure applied (See
Table 6). Note, however, that many of the studies presented in
Table 6 used low-viscosity oils, whereas our emulsion used high-viscosity kitchen oil, which can cause membrane fouling immediately. As such, many of these studies cannot be compared directly with ours but are presented for information purposes only.